Summary of Contents for CSO VEGA
- Page 1 Strumenti Oftalmologici Instructions for Use and Maintenance Mod. 10mW LED-based UV Emitter CSO Srl - Costruzione Strumenti Oftalmici...
-
Page 2: Table Of Contents
PROTECTION FROM DUST ............................ 22 REPLACING THE LINE FUSES ..........................22 PERIODIC SAFETY CHECKS ....................... 23 END-OF-LIFE DISPOSAL INFORMATION ................24 LIABILITY ............................25 Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 2 of 25 10mW... -
Page 3: Description And Use
DESCRIPTION AND USE VEGA is a medical electrical device designed and built by CSO Srl. It consists of a LED light source that radiates in the ultraviolet spectrum (UV-A). The instrument is designed to be used exclusively for treatment of disorders of the cornea, in an ophthalmologic clinical setting and only by expert specialized physicians. -
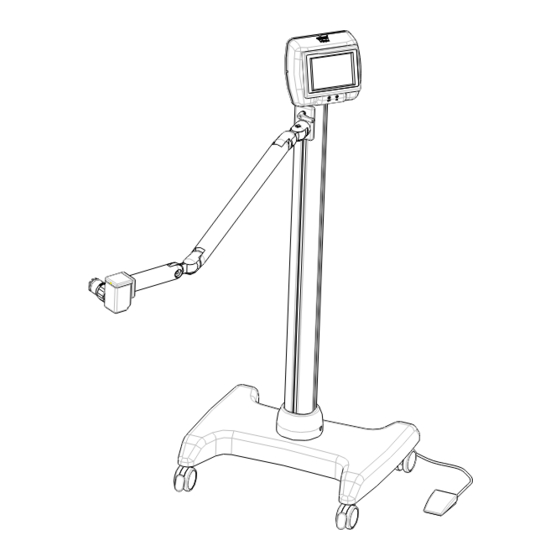
Page 4: Standard Components
5- FOOT SWITCH Standard accessories: - Power cord - Wrenches for assembly - UV light meter - Adapter holder - This use and maintenance manual. Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 4 of 25 10mW... -
Page 5: Ambient Conditions For Shipping, Warehousing, And Use
700 hPa and 1060 hPa, and relative humidity between 30% and 75%. DATA PLATES AND LABELS C.S.O. srl Via degli Stagnacci, 12/E 50010 SCANDICCI (FIRENZE) - ITALY UV EMITTER Mod. VEGA SERIAL NUMBER …………………. INVISIBLE RADIATION DO NOT VIEW DIRECTLY WITH OPTICAL INSTRUMENTS... - Page 6 CONTROL BOX Emission power: 10 mW FUSES 2 x 315 mA - 5x20 - T type (IEC 127) Wavelength: 370 nm Mod. VEGA 230-240 V 18VA FUSES 2 x 160 mA - 5x20 - T type (IEC 127) Bandwidth: 8 nm FREQ.
- Page 7 Notice of duty to separately collect and dispose of end- instruction manual carefully before installing, commissioning, of-life electrical and electronic equipment (see section on this and using the instrument. topic). Ground connection. Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 7 of 25 10mW...
-
Page 8: Commands And Signals
2.1- ARM LOCK 3.1- UV EMISSION INDICATOR (yellow light) 3.2- MALFUNCTION INDICATOR (red light) 3.6- MAIN SWITCH 3.9- CONTROL BOX ROTATION LOCK 5- FOOT SWITCH Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 8 of 25 10mW... -
Page 9: Installation And Commissioning
Please be sure to hold the arm in the lower position with the final part 90° tilted as indicated in the picture below 90° Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 9 of 25 10mW... -
Page 10: Assembly
Insert the optical head (3) onto the column (4). Adjust rotation friction by tightening the screw (4.2) with the wrench supplied for that purpose. Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 10 of 25... - Page 11 Lock in place with the wrench supplied for that purpose. (1.1) Adjust the optical head in the horizontal position and lock tightly the two screw 34 and 36 on the mechanism 2.3 Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 11 of 25...
-
Page 12: Connections
Avoid damp and dusty locations and locations subject to brusque changes in temperature and humidity. Disconnect the instrument from the mains supply socket before cleaning and/or disinfecting. Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 12 of 25 10mW... - Page 13 Electrical Systems” and is fully compliant with these standards. Note that the standard version unit can be connected to other instruments, medical electrical and not; CSO cannot test the compliance of all possible system configurations. Any additional accessories (video recorder, monitor, or similar equipment) connected to the analog or digital interfaces must each be certified in accordance with the respective pertinent standards.
-
Page 14: Power Emission Check
Before taking any reading, allow a few minutes’ time on-site for instrument temperature to stabilize. Replace the batteries immediately whenever the “BAT” Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 14 of 25 10mW... - Page 15 If the instrument is unused for a long period of time, remove the batteries. Do not open the instrument or tamper with its parts. Control and calibration may be performed only at CSO. For further information regarding the UV meter, refer to the original instruction manual.
-
Page 16: Instructions For Use
Correct focusing ensures correct UV emission power density for meeting medical procedure guidelines (10 mW/cm²) only at this focus point. OPTICAL HEAD LUMINOUS SPOTS FOR FOCUSING Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 16 of 25 10mW... -
Page 17: Display Functions
Step 1 is the UV- treatment phase that will be finished within 9 minutes – the system will switch off automatically after 9 minutes TIME (3.8.3)- The elapsed time for each step is displayed in minutes and seconds. STATUS (3.8.4)- Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 17 of 25 10mW... -
Page 18: Treatment Procedure
Iontophoresis 5’ Press the foot switch to start step 1, The procedure will be finished in 9 minutes, the system will switch off automatically Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 18 of 25... -
Page 19: Technical Features
Minicamera, ¼”, color, model ECH-3030ST Display monitor, 5.6”, color, model SJD-56S Monitor Video out 75-Ohm PAL video-composite output 1260 – 545 – 1360 mm Dimensions Weight 20 kg Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 19 of 25 10mW... -
Page 20: Regulatory Compliance Information
REGULATORY COMPLIANCE INFORMATION The VEGA is designed and built in compliance with EU Directive 93/42/EEC and the following harmonized standards: CEI EN 60601-1:1991 “Medical Electrical Equipment – Part 1: General Requirements for Safety" as amended. CEI EN 60825-1:2003 – “Safety of Laser Products - Part 1: Equipment Classification, Requirements, and User Guide.”... -
Page 21: Equipment Lifecycle
For reasons of prudence, the working life of the device is therefore set at ten years from the date of commissioning (re: Annex I – Directive 93/42/EEC). Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 21 of 25... -
Page 22: Routine Maintenance
Replace the voltage-changer/fuse-holder box. checks, periodic safety Plug in the instrument. checks, etc.) is the exclusive competence of the CSO technical assistance service. In the event of request for assistance, the version of firmware and the operating... -
Page 23: Periodic Safety Checks
CSO srl. Attention! The Mod. VEGA instrument is designed and built in full compliance with applicable laws and standards and must pass rigorous safety The periodic safety checks testing before leaving the factory. -
Page 24: End-Of-Life Disposal Information
Current legislation provides severe sanctions in the case of failure to respect disposal laws and regulations in force. Mod. VEGA - Instructions for Use and Maintenance - rev. 8- Page 24 of 25 10mW... -
Page 25: Liability
the electrical system of the installation site does not comply with CEI standards and the pertinent laws and regulations in force. CSO srl also declines any and all responsibility for direct or indirect consequences and/or damage to persons and/or things deriving from improper use of the instrument and/or from erroneous clinical evaluation of information derived from its use.











Need help?
Do you have a question about the VEGA and is the answer not in the manual?
Questions and answers