Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for mectron Multipiezo
- Page 1 USE AND MAINTENANCE MANUAL multipiezo 0051...
- Page 2 O-RING 6,5X2 NBR...
- Page 3 Copyright © Mectron S.p.A. 2022. All rights reserved. No part of this document may be reproduced, in any form whatsoever, without the prior written consent of the copyright holder.
-
Page 4: Table Of Contents
TABLE OF CONTENTS Introduction Intended Use Description of the Device Disclaimer Safety Requirements Symbols Identification Data 2.1 Identification Plate of the Device 2.2 Handpiece Identification Data 2.3 Inserts Identification Data Delivery List of Components Installation First Installation Safety Requirements During Installation Connecting the Accessories 5.1 Power On/Off Description of the Keyboard... - Page 5 11.1 Electromagnetic Compatibility IEC/EN 60601-1-2 11.1.1 Guide and Manufacturer’s Declaration - Electromagnetic Emissions 11.1.2 Accessible Parts of the Casing 11.1.3 Guide and the Manufacturer's Declaration - Electromagnetic Immunity 11.1.3.1 Power Connection BC Input 11.1.3.2 Points of Contact with the Patient 11.1.3.3 Parts Accessible to the Input / Output Signals 11.1.4 Specifications of the Tests for the Immunity of the Accessible Parts of the Casing to the Wireless RF Communications Device Troubleshooting 12.1 Diagnostic System and Symbols on the Keyboard 12.2 Quick Troubleshooting 12.3 Replacing the Fuses 12.4 Customer Service - Returns and/or Repairs 12.4.1 Repairs 12.4.2 Returned Goods Warranty...
- Page 6 PAGE LEFT INTENTIONALLY BLANK...
-
Page 7: Introduction
NOTE: Identifies special information to clarify or emphasize important instructions. Intended Use multipiezo is a piezoelectric ultrasonic dental scaler intended for use, with the appropriate associated tip inserts, in the following dental applications: • Scaling: all general procedures for • Endodontics: all treatments for root... -
Page 8: Description Of The Device
• Assembly operations, extensions, re- The manufacturer MECTRON shall be under adjustments, upgrades and repairs of the no liability, expressed or implied, for any type device are carried out by personnel not of personal injury and/or property damage, authorised by Piezosurgery Inc.;... -
Page 9: Safety Requirements
• Incorrect/omitted maintenance on page 66 this manual. compared to what is stated in Chapter 9 Safety Requirements WARNING: Contraindications. WARNING: Contraindications. Do not use the multipiezo on patients who Interference with other equipment. carry heart stimulators (Pace-makers) or Even if compliant with standard IEC 60601- other implantable electronic devices. This 1-2, multipiezo may interfere with other precaution also applies to the operator. devices in the vicinity. The user should take whatever steps are necessary to eliminate WARNING: Contraindications. - Page 10 CAUTION: In case the end user, when WARNING: Control of Infections. operating in his or her own medical study • First Use. The reusable or clinic, must subject the electro-medical accessories(brand new or returned by equipment and systems to periodical service) and the single use accessories inspections in order to adhere to imposed...
- Page 11 INTRODuCTION NOTE: In case of accident attributable to the WARNING: Breakage and wear-out of device during correct use and in accordance the inserts. High frequency oscillations and with the intended use, a report must be wear-out may, in rare circumstances, lead to made to the Competent Authority and the breakage of the insert. manufacturer indicated on the product label.
-
Page 12: Symbols
Symbols Symbol Description Symbol Description Device compliant Nemko brand with Regulation (Eu) Compliance with uL - 2017/745. Notified 0051 CSA regulations body: IMQ S.p.A. Medical device Caution! Consult instructions for use or consult Manufacturer electronic instructions for use Date of manufacture Serial number Batch number Product code... -
Page 13: Identification Data
Always provide this information every time After Sales Service to provide fast and efficient that you contact Piezosurgery Inc.’s Service. Identification Plate of the Device Each device equipped with identification plate indicating the main manufacturer Mectron S.p.A. technical characteristics serial Via Loreto 15/A 16042 Carasco -GE- Italy number. The identification plate is located multipiezo on the bottom of the device’s console. 100-240 V 90 VA - 50/60 Hz. -
Page 14: Handpiece Identification Data
Chapter 1.5 on page 6. 107000000 03120238 0476 0051 YYYY-MM MANIPOLO ABLATORE SLIM MANIPOLO ABLATORE 114000000 SLIM Data Matrix HIBC 128 03120238 Inserts Identification Data The name of the insert itself (Reference 1), MECTRON logo (Reference 2), and insert batch number (Reference 3) are laser-marked on each insert. -
Page 15: Delivery
List of Components See the Figure on the inside cover. 3 on page 10), and accessories that can be multipiezo is supplied with the standard ordered separately (see Table 4 on page 10). equipment (see Table 2 on page 9), a set NOTE: Both the items included in the... - Page 16 Accessories available for order with standard supply Item Code Description Ref. Torque wrench 02900137 K10 torque wrench Insert tips 0296xxxx "S” series reusable scaler inserts “PE” series reusable scaler inserts 0308xxxx 0305xxxx “R” series reusable scaler inserts “ER” series reusable scaler inserts 0345xxxx 0235xxxx “E” series reusable scaler inserts “D” series reusable scaler inserts 0299xxxx “CM”...
-
Page 17: Installation
WARNING: To avoid any risk of electric of the equipment and multipiezo, in the shock this device must be grounded. Only configuration in which they will be used, connect the console to hospital grade should be verified prior their use. receptacle to ensure electrical grounding... -
Page 18: Connecting The Accessories
WARNING: Risk of explosion. CAUTION: The device can be transported The device cannot operate in environments but must be handled with care when moved. where there are saturated atmospheres of Position the pedal on the floor so that it flammable gases (aesthetic mixtures, oxygen, can only be activated intentionally by the etc.). operator. WARNING: Install the device in a safe CAUTION: Before connecting the... - Page 19 CAUTION: Check that the male coupling on the device core unit is clean and that its O-rings are not worn. NOTE: Use the support only to install the Mectron 500 ml tank and to house the handpiece. Do not use the support for other purposes. Keep the tank in a vertical position and push it towards the unit’s core until it is firmly connected;...
-
Page 20: Use
Correctly connect the scaler handpiece onto its cord by matching the alignment notch on the handpiece connector with the alignment key on the cord connector. Check that the electrical contacts of both are perfectly dry and if necessary dry them by blowing with compressed air; Position the handpiece on its support. NOTE Use the support only to install the Mectron 500 ml tank and to house the handpiece. -
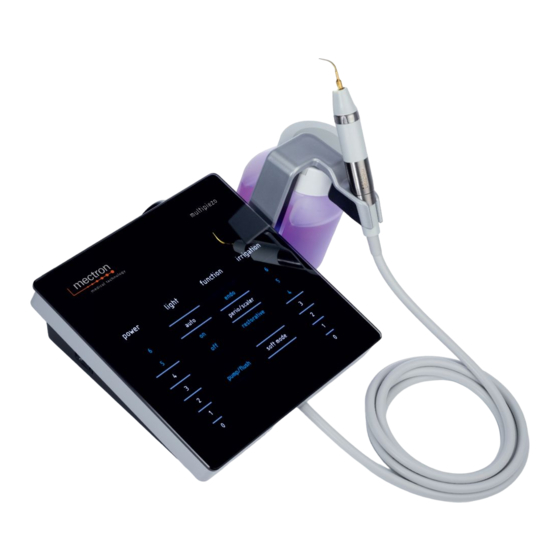
Page 21: Description Of The Keyboard
NOTE: By default, when the console is CAUTION: Always position the device turned on the device is programmed on the in way so that the power switch is easily following settings: reachable, since this switch is considered as a load-break switch. •... - Page 22 FILLING AND FLUSHING THE IRRIGATION CIRCUIT (Ref. 2 inside cover) The device is equipped with the “pump/ flush” key, which depending on the operating mode allows running either the PuMP or the FLuSH function. The PuMP function can be used at the beginning of the treatment, to prime the entire irrigation line up to the insert, so...
- Page 23 LIGHT (Ref. 3 inside cover) Depending on the type of treatment that needs to be carried out, 3 possible options can be selected from the “light” list: • If the AUTO option is selected, the LED light on the handpiece nose cone lights up when the pedal is pressed and automatically switches off 3 seconds after the pedal is released;...
- Page 24 FUNCTIONS (Ref. 4 inside cover) Depending on the type of operation, it is possible to select one of 3 options from the “function” list, as follows: • ENDO: dedicated to endodontic treatments such as root canal cleaning and the retrograde approach. • PERIO/SCALER: dedicated...
- Page 25 0 only with the power set from 1 to 5. Selecting RESTORATIvE and power on 6 the irrigation is compulsory. SYMBOLS (Ref. 7 inside cover) multipiezo is provided with a diagnostic circuit that allows the detection of malfunctions and viewing of their type on the touch keyboard by means of a perio/scaler perio/scaler symbol.
-
Page 26: Safety Requirements Before And During Use
The ultrasonic vibration could cause procedures described in the Chapter 8 decementation/loosening of such artifacts. on page 31 WARNING: Contraindications. • Every use. Once used, each reusable Do not use multipiezo on patients who accessory must be thoroughly carry heart stimulators (Pace-makers) or reprocessed prior to reuse, according to other implantable electronic devices. This the procedures described in the Chapter precaution also applies to the operator. - Page 27 If a drop in performance occurs, see to its the the treatment is correctly screwed onto replacement. the handpiece. Only use the Mectron torque The state of wear of the most common wrench, supplied with the device, to fasten inserts (S1, S1-S, S2, S5, P2, P4, P10) can be the insert to the handpiece. Do not use any checked using the supplied INSERT-CARD. To...
-
Page 28: Instructions For Use
WARNING: Handle sharp-edged and WARNING: Pay attention to the pointed inserts with particular care. During positioning of the pedal, which must be the tightening/untightening operations, positioned on the floor so that it can only be the sharp-edged/pointed parts of the insert activated intentionally by the operator. could cause harm. WARNING: Do not use the device in case of breaks or cracks on the casing. Instructions for Use After having connected all the accessories as described in Chapter 4.3 on page 12... - Page 29 Screw in the preselected insert on the multipiezo handpiece until it comes into contact; Tighten the insert using the Mectron torque wrench. For correct use of the Mectron torque wrench, proceed as follows: •...
-
Page 30: Important Information On The Inserts
Select the type of function, power and irrigation necessary and the light if desired, on the keyboard; Lift the handpiece and press the pedal to start treatment; At the end of treatment stow the scaler handpiece on its support. CAUTION: Owing to its conformation, the handpiece can rotate. - Page 31 Mectron inserts are used. use of non- breakage, create a smooth path with original Mectron inserts may result in a manual endo file and plan an access patient or operator injury or system as straight as possible to limit creases malfunction and will void any applicable in the insert.
-
Page 32: Flush Function
FLUSH FUNCTION The FLuSH function allows to run a flushing cycle on the irrigation circuit of the handpiece used during the treatment, by following the steps described in this chapter. CAUTION: FLUSH function. The FLuSH CAUTION: Failure to carry out flushing function must be used after every treatment, of the handpiece tubing will lead to salt before starting the cleaning and sterilization crystallisation that can seriously damage the procedures. - Page 33 FLuSH FuNCTION To enter the flushing mode, select “pump/ flush” menu on the touch keyboard: all the other selection options present on the display are disabled; NOTE: The “flush” mode can be interrupted at any time by pressing the perio/scaler perio/scaler restorative restorative “pump/flush” key again. The keyboard will be reactivated and configured with the last setting used. To start the “pump/flush” function while the writing “pump/flush” is flashing, press the pedal once and release it:...
-
Page 34: Disassembling Parts For Cleaning And Sterilisation
DISASSEMBLING PARTS FOR CLEANING AND STERILISATION Before carrying out the cleaning procedures described in Chapter 8 on page 31 , disconnect all accessories and components of multipiezo. WARNING: Switch the device off. Always switch the device off using the switch and disconnect the power supply cable from the wall socket and from the device core unit before carrying out cleaning and sterilisation tasks. If present, remove the insert from the... - Page 35 DISASSEMBLING PARTS FOR CLEANING AND STERILISATION Disconnect the handpiece from its cord; CAUTION: Do not attempt to unscrew or turn the connector when disconnecting the handpiece. The connector could get damaged. Unscrew the nose cone from the handpiece; NOTE: The nose cone has a light pipe. If the nose cone is unscrewed, the light pipe will no longer be held in place and may slide and be disconnected.
- Page 36 Disconnect the tank from the device core unit, pulling it outwards; Disconnect the pedal from the device: grab the pedal connector, press the release clip and pull the connector back; CAUTION: Do not attempt to unscrew or twist the connector during the disconnection: the connector could get damaged. CAUTION: When disconnecting the foot pedal, always and only hold the connector of the cord.
-
Page 37: Cleaning And Sterilisation
Drying and Drying lubrication Lubrication Sterilisation Packaging, Sterilisation and Storage NOTE: Repeated reconditioning has a minimal effect on the devices and their accessories. The end of the service life of the accessories is generally determined by wear or damage resulting from use. Mectron guarantees the integrity of its sterilisable scaler handpieces for up to 250 reconditioning cycles. -
Page 38: Preparation
8.1 Preparation Run the flush function (see Chapter 6 WARNING: Always switch off the device on page 26 using the O/I switch and disconnect it from the power mains before carrying out cleaning Check that all of the following tasks. accessories have been removed/ disconnected from the machine core CAUTION: Always disconnect the insert unit (see Chapter 7 on page 28 from the handpiece before cleaning and sterilising it. - Page 39 CLEANING AND STERILISATION Dry the parts using a dry, non-abrasive, low-lint cloth. CAUTION: Do not sterilise the parts CAUTION: Do not use running water to in question. They may stop working clean the parts in question. and cause damage to people and/or CAUTION: Do not soak these parts in property. liquid and/or various kinds of solutions. WARNING: Always switch off CAUTION: If disinfection is required, the device using the O/I switch and water-based disinfectant solutions must be disconnect it from the power mains...
-
Page 40: Cleaning The Tank And Cap
8.3 Cleaning the Tank and Cap The following procedure must be carried out on the tank and cap of the device. 8.3.1 Preparation Disconnect the tank from the machine CAUTION: Do not sterilise the tank and Chapter 7 on page 28 core unit (see cap in an autoclave. They could be damaged. unscrew the cap from the tank. 8.3.2 Required Materials • Water; • Products containing sodium hypochlorite; • Cleaning solution (pH 6-9); • Products containing hydrogen peroxide; • Clean, soft, low-lint cloth; • Products containing abrasive substances; • Demineralised water. • very acid products (PH < 4); CAUTION: To disinfect the device and/ • Products containing aldehyde, amine or its accessories, it is recommended the use and/or phenols;... - Page 41 CLEANING AND STERILISATION Clean the external and internal surfaces of the tank and cap with a clean, soft, low-lint cloth, dampened with a mild detergent solution (pH 6-9); Thoroughly rinse the inside and outside of the tank and cap under running water to eliminate all residue of the detergent solution; Remove any residue on the external or internal surfaces of the tank and cap using a soft, low-lint cloth, dampened with demineralised water;...
-
Page 42: Cleaning The Sterilisable Accessories
8.4 Cleaning the Sterilisable Accessories NOTE: The cleaning procedures must be CAUTION: The instructions supplied performed immediately after each use. below have been validated by the Immerse the insert and/or instrument in manufacturer of the medical device as ABLE demineralised water or in an enzymatic to prepare a medical device for re-use. detergent solution immediately after use. The process manager is responsible for Do not leave residue or blood deposits on... -
Page 43: Scaler Handpiece
CAUTION: Once used, dispose of the enzymatic detergent correctly, do not recycle it. a) Process validated by independent bodies with Mectron ENZYMEC enzymatic detergent, 0.8% v/v. 8.4.1.2 Scaler Handpiece Clean the surface of the handpiece and of its connector using a clean, soft, lint free, cloth moistened with the prepared... - Page 44 Use the enzymatic detergent solution and a clean soft bristled, nylon brush to gently scrub the external surface of the handpiece and the front terminal until all visible soil has been removed. The following parts must be brushed meticulously: •...
-
Page 45: Inserts
CLEANING AND STERILISATION Thoroughly rinse the front terminal under running warm tap water and then under demineralized water to eliminate any residual detergent; Hold the handpiece with its front end pointed downward; Rinse thoroughly the front end and the surfaces of the external body of the handpiece under running warm tap water to eliminate any residual detergent; FINAL RINSING Hold the handpiece with its front end pointed downward;... - Page 46 While soaking in the enzymatic solution, gently brush all the surfaces until all visible soil has been removed. Use a clean brush with soft nylon bristles for the external surfaces, and a clean pipe cleaner with soft nylon bristles for the internal cavities and grooves.
- Page 47 CLEANING AND STERILISATION Rinse the internal channel of the insert with demineralised water at a pressure of 3.8 bar for at least 10 seconds so as to eliminate any soil residue.; x10 sec. H₂O Place the insert in the ultrasonic tank immersed in the enzymatic detergent solution at 24 °C ±2 °C and run a cycle for at least 20 minutes;...
-
Page 48: Inserts Torque Wrench
Rinse the insert(s) in demineralised water; Rinse the internal channel of the insert with demineralised water at a pressure of 3.8 bar for at least 10 seconds so as to eliminate any soil residue. x10 sec. H₂O 8.4.1.4 Inserts Torque Wrench Place the torque wrench in a clean container, in a horizontal position. - Page 49 CLEANING AND STERILISATION While soaking in the enzymatic solution, gently brush all the surfaces until all visible soil has been removed. Use a clean brush with soft nylon bristles for the external surfaces, and a clean pipe cleaner with soft nylon bristles for the internal cavities and grooves. NOTE: Thoroughly brush all the following parts of the torque wrench for about 20 seconds:...
-
Page 50: Cleaning Check
Remove the torque wrench from the ultrasonic tank and rinse it under running water so as to eliminate all detergent residue. Brush the internal and external surfaces of the wrench with a clean brush with soft nylon bristles, under running water. 8.5 Cleaning Check 8.5.1 Required Materials •... - Page 51 CLEANING AND STERILISATION Repeat the control operations for the other accessories (inserts, tightening wrenches), repeating if necessary the cleaning cycle.
-
Page 52: Drying And Lubrication
8.6 Drying and Lubrication 8.6.1 Required Materials • Compressed air; • FDA approved medical grade lubricant. • Soft, low-lint cloth; Thoroughly dry all parts of the scaler handpiece, scaler nose cone, and light pipe by blowing them with compressed air; CAUTION: The scaler handpiece electrical contacts must be dry at the start and end of the sterilisation cycle, before the cord is connected to the device. Always make sure that the... -
Page 53: Sterilisation
CLEANING AND STERILISATION Dry the insert tips torque wrench using a soft cloth with low fibre release; Lubricate the insert tips torque wrench with approved medical-grade lubricants by spraying it directly onto the peripheral contact surface inside the torque wrench, as indicated in the figure. CAUTION: Do not use oil or silicone- based lubricants. After having applied the lubricant, remove any excess oil using a clean lint- free cloth. - Page 54 Seal the inserts individually inside a disposable bag for sterilisation. Seal the wrench individually inside a disposable bag for sterilisation.
-
Page 55: Sterilisation Method
The sterilisation process validated by Mectron WARNING: use exclusively a pre- vacuum autoclave to sterilize the multipiezo S.p.A., in a pre-vacuum autoclave, guarantees sterilizable intruments. a SAL 10 by setting the parameters indicated below: CAUTION: Never use any other •... -
Page 56: Maintenance
WARNING: Periodically check the Disconnect the device from the mains; integrity of the power supply cable; if In case of long periods of non-use, damaged, replace it with an original Mectron place the device back in its original spare part. packaging, in a safe place; 9.1 Replacing the Peristaltic Pump On the back of the device there is the plastic cover which covers the housing of the peristaltic pump. - Page 57 MAINTENANCE Remove the two tubes of the pump from the respective clutches positioned below Extract the peristaltic pump from its base, pulling it towards yourself; Attach the new peristaltic pump onto the base until you hear it click in, and connect the two pump tubes to the respective couplings underneath it;...
-
Page 58: Methods And Precautions For Disposal
METHODS AND PRECAUTIONS FOR DISPOSAL WARNING: Hospital waste. Treat the multipiezo must be disposed of and treated as following objects as hospital waste: waste subject to separate collection. Failure to comply with the previous points can • Inserts, when worn or broken; result in a penalty pursuant to the directive •... - Page 59 TECHNICAL DATA Adjustable using the touch screen 7 flow rate levels: from 0 (0 ml/min) to 6 (approx. 28 ml/min) (see Chapter 5.2 on page 15 Peristaltic pump flow rate Tank capacity: 500 ml. Tank lighting system: Blue LED light power risk free according to standard IEC/EN 62471. Light function set to AuTO: The handpiece LED lights up as soon as the machine starts working, and turns off 3 seconds after the pedal is released Light function set to ON: The handpiece LED is always lit; after 100 seconds of non-...
-
Page 60: Guide And Manufacturer's Declaration - Electromagnetic Emissions
11.1.1 Guide and Manufacturer’s Declaration - Electromagnetic Emissions multipiezo is designed to operate in the electromagnetic environment specified below. The purchaser or user of multipiezo should ensure that it is used in such an environment. Emissions Test Compliance Electromagnetic Environment Guidance multipiezo uses RF energy only for its internal RF Emissions operation. Therefore, its RF emissions are... -
Page 61: 11.1.2 Accessible Parts Of The Casing
Only applicable to equipment and systems with physiological signal simulation and multipiezo, must magnetically sensitive components or circuits. be positioned within a 0.1 m radius of the vertical e) During the tests, multipiezo can be supplied with any plane of the uniform field area in the same direction NOMINAL input voltage, but with the same frequency as multipiezo. as the test signal. -
Page 62: Guide And The Manufacturer's Declaration - Electromagnetic Immunity
11.1.3 Guide and the Manufacturer's Declaration - Electromagnetic Immunity 11.1.3.1 Power Connection BC Input multipiezo is designed to operate in the electromagnetic environment specified below. The purchaser or user of multipiezo should ensure that it is used in such an environment. Essential EMC Electromagnetic Phenomenon standard or test Immunity test values Environment Guidance method The quality of the network Electrical fast ±2 kv on contact voltage should be that of a transient/burst IEC 61000-4-4 100 KHz repetition typical commercial or hospital... - Page 63 The test can be performed at any supply voltage j) Devices and systems that do not have a surge within the multipiezo range of NOMINAL voltages. protection device in the primary power circuit can If multipiezo is tested at one supply voltage value, it only be tested at ± 2 kv between the line(s) and the need not be retested at other voltage values. ground (common mode) and at ± 1 kv between line(s) and line(s) (differential mode).
-
Page 64: 11.1.3.2 Points Of Contact With The Patient
11.1.3.2 Points of Contact with the Patient multipiezo is designed to operate in the electromagnetic environment specified below. The purchaser or user of multipiezo should ensure that it is used in such an environment. Essential EMC Electromagnetic Phenomenon standard or test Immunity test values Environment Guidance method The floor must be made of wood, concrete or ceramic Electrostatic ±8 kv on contact tiles. If floors are covered with discharges IEC 61000-4-2 ±2 kv, ±4 kv, ±8 kv, synthetic material, the relative (ESD) ±15 kv in air humidity should be at least 30%. Portable and mobile RF communication devices should not be used near any Conductive 0.15 MHz - 80 MHz... -
Page 65: 11.1.3.3 Parts Accessible To The Input / Output Signals
TECHNICAL DATA 11.1.3.3 Parts Accessible to the Input / Output Signals multipiezo is designed to operate in the electromagnetic environment specified below. The purchaser or user of multipiezo should ensure that it is used in such an environment. Essential EMC Electromagnetic Phenomenon standard or test Immunity test values Environment Guidance method The floor must be made of wood, concrete or ceramic Electrostatic ±8 kv on contact tiles. If floors are covered with discharges IEC 61000-4-2 ±2 kv, ±4 kv, ±8 kv, synthetic material, the relative (ESD) ±15 kv in air humidity should be at least 30%. The quality of the network Electrical fast ±1 kv on contact voltage should be that of a... -
Page 66: Specifications Of The Tests For The Immunity Of The Accessible Parts Of The Casing To The Wireless Rf Communications Device
11.1.4 Specifications of the Tests for the Immunity of the Accessible Parts of the Casing to the Wireless RF Communications Device multipiezo is designed to operate in an electromagnetic environment in which radiated RF disturbances are under control. The purchaser or operator of multipiezo can help prevent electromagnetic interferences by guaranteeing a minimum distance between the mobile and portable RF communication devices (transmitters) and multipiezo, as recommended below, in relation to the maximum output power of the radio communication devices. -
Page 67: Troubleshooting
Otherwise, there may be a performance degradation of these devices. TROUBLESHOOTING 12.1 Diagnostic System and Symbols on the Keyboard multipiezo is provided with a diagnostic circuit that allows the detection of malfunctions and viewing of their type on the touch keyboard by means of a symbol. users, by using the following table, are guided to identifying and the possible resolution of the malfunction detected. Symbol on keyboard Possible cause Solution... - Page 68 Symbol on keyboard Possible cause Solution Check that there are no impediments for the rotation of the pump. Peristaltic pump malfunction Check that the pump and the two pipes are correctly installed. The device has been Turn off and wait for 5 switched off and on again seconds before switching on without waiting 5 seconds the device again. Turn off and wait 5 seconds Faults on the electrical before switching the device...
-
Page 69: Quick Troubleshooting
Unscrew the insert and screw The insert is not correctly it back in correctly using the During operation faint tightened on the handpiece Mectron torque wrench (See whistling noise Chapter 5.4 on page 22 heard coming from Fill the irrigation circuit using multipiezo handpiece. - Page 70 Replace the peristaltic pump The peristaltic pump is worn (See Chapter 9.1 on page Unscrew the insert and screw The insert is not correctly it back in correctly using the tightened on the handpiece Mectron torque wrench (See Poor performance Chapter 5.4 on page 22 Insert broken, worn or Replace the insert with a deformed new one Table 7 – Quick Troubleshooting...
-
Page 71: Replacing The Fuses
TROuBLESHOOTING 12.3 Replacing the Fuses WARNING: Switch the device off. Always switch the device off using the main switch and disconnect it from the power supply socket before carrying out the next intervention. Pry with a flat screwdriver, inserting the tip into the seat of the fuse holder compartment located under the power socket; Extract the fuse holder compartment; WARNING: Replace the fuses in respect of the characteristics indicated in Chapter 8 on page 31... -
Page 72: Customer Service - Returns And/Or Repairs
12.4 Customer Service - Returns and/or Repairs If you need technical assistance regarding the Please provide the following information: use, or you encounter a problem that requires • Data of the owner with telephone servicing or repair, contact PIEZOSuRGERY ® number; Inc. Customer Service at (1.888.87-PIEZO). • Product name; Returning products for any reason, requires •... -
Page 73: Warranty
WARRANTY WARRANTY Any non-approved usage of the multipiezo covered by warranty by providing , at its will void the warranty. option, one of the following: service or repair Any usage of non-Mectron parts, tips, of the product, or a replacement of the components or procedures will void the product. - Page 76 Distributed in U.S. by: Manufacturer: Mectron S.p.A. 850 Michigan Avenue via Loreto 15/A Columbus, Ohio 43215 16042 Carasco (Ge) Italy Tel. +39 0185 35361 Phone 614.459.4922 Fax 614.459.4981 Fax +39 0185 351374 www.piezosurgery.us www.mectron.com e-mail: mectron@mectron.com e-mail: info@piezosurgery.us Reseller...
















Need help?
Do you have a question about the Multipiezo and is the answer not in the manual?
Questions and answers