Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for mectron PIEZOSURGERY GP
- Page 1 USE AND MAINTENANCE MANUAL 0051...
- Page 2 NOT SUPPLIED...
- Page 3 Piezosurgery GP is manufactured and sold internationally by Mectron S.p.A. under the name Piezosurgery white...
- Page 5 Copyright © Mectron S.p.A. 2021. All rights reserved. No part of this document can be reproduced in any form without the written consent of the copyright owner.
-
Page 6: Table Of Contents
TABLE OF CONTENTS Introduction Intended Use of PIEZOSURGERY® GP Description of the Device 1.2.1 Users Disclaimer Safety Precautions Symbols Identification Data 2.1 Device Identification Label 2.2 Handpiece Identification Data 2.3 Inserts Identification Data Delivery List of the Components of PIEZOSURGERY® GP Installation Safety Requirements in the Installation Phase Connecting the Accessories 5.1 Switching the Device On and Off... - Page 7 PAGE INTENTIONALLY LEFT BLANK...
- Page 8 PAGE INTENTIONALLY LEFT BLANK...
-
Page 9: Introduction
• check for any available updates in CAUTION: (Identifies conditions or section MANUALS of MECTRON practices that could result in minor injury or website device damage) • contact Piezosurgery Inc. Customer Service. -
Page 10: Description Of The Device
The manufacturer Mectron and the distributor, Piezosurgery Inc. shall be under no liability, expressed or implied, with respect to any damages (personal injury and/or damage... -
Page 11: Safety Precautions
Piezosurgery/Mectron inserts; Use of non-original Piezosurgery/ 10. Lack of stock materials (handpiece, Mectron inserts, used in accordance inserts, wrenches) to be used in the to designed and tested settings event of device stop due to fault or of of Piezosurgery/Mectron original inconveniences. - Page 12 CAUTION: Contraindications. Allow intended for single use only. reusable, autoclavable items to gradually WARNING: Only use original return to room temperature after steam Piezosurgery/Mectron inserts, accessories, sterilization and prior to usage. The cooling and spare parts. process must not be accelerated. CAUTION: No modification of this equipment is allowed.
-
Page 13: Symbols
INTRODUCTION Symbols Symbol Description Symbol Description Device compliant with Regulation (EU) Nemko Mark 2017/745. Notified UL CSA compliance 0051 body: IMQ S.p.A. Caution, read the Medical Device Instructions For Use Operating Instructions Manufacturer Date of manufacture Serial Number Lot Number Product Number Single-Use Do not re-sterilize... - Page 14 Symbol Description Symbol Description Do not touch moving General warning parts Temperature limitation Humidity limitation for for transport and transport and storage storage Atmospheric pressure Do not use if package is limitation for transport damaged and storage International Protection International Protection IP22 Code of the mechanical IP20...
-
Page 15: Identification Data
Service to answer the inquiries quickly and contacting Piezosurgery Inc. Customer Service. Device Identification Label NOTE: PIEZOSURGERY® GP is manufactured and sold internationally by Mectron S.p.A. under the name PIEZOSURGERY® white. Every device has an identification label that reports the main technical manufacturer Mectron S.p.A. -
Page 16: Inserts Identification Data
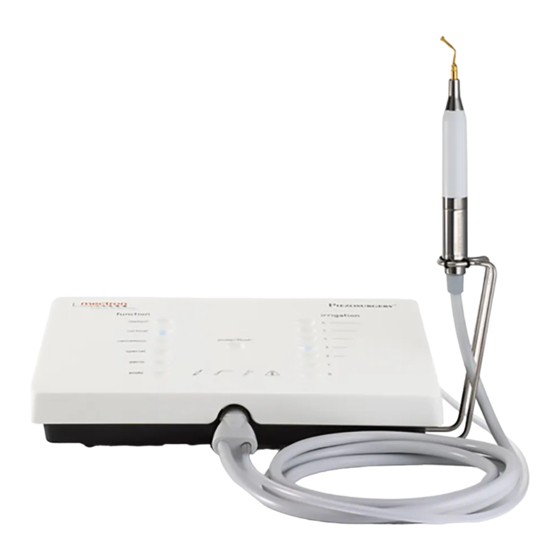
03120217 HIBC 128 Data Matrix white PIEZOSURGERY ® white Figure 1 – Handpiece Identification Data Inserts Identification Data The following data are laser-marked on each insert: • the name of the insert (Ref. 1); • the Mectron logo (Ref. 2); • and the lot number to which the insert belongs (Ref. 3);... -
Page 17: Delivery
Foot pedal with cable and connector; All material shipped by Piezosurgery Inc. is controlled at the time of dispatch. Back-up foot pedal; The device is shipped appropriately protected K8 Mectron torque wrench; and packaged. Peristaltic pump tubing kit (The kit is Upon receipt of the device, check for any composed by a set of 6 peristaltic pump possible damage caused during transport and tubing);... -
Page 18: Installation
INSTALLATION The device must be installed in a suitable and plug is always, easily reachable, since this plug convenient place for its use. is considered as a disconnecting means. Place the console on a sturdy, flat ,dry and Take care to ensure that cables do not hinder horizontal surface. -
Page 19: Connecting The Accessories
CAUTION: Use original Piezosurgery/ Mectron peristaltic pump tubing only, as damage or substandard performance could result. With the writing UP facing upward, insert the connector of the handpiece cord into the handpiece receptacle on the... - Page 20 Fit the peristaltic pump tubing into the peristaltic pump proceeding as follows: • Open completely the peristaltic pump cover; • Insert the tubing by placing it over the pump rollers; • Close the peristaltic pump cover completely; WARNING: Do not operate the footswitch of the PIEZOSURGERY®...
- Page 21 INSTALLATION Connect the foot pedal to the back of the device in the socket marked with the symbol , using the foot pedal cable plug, ensuring it clicks into place; CAUTION: The user must pay particular attention to the foot pedal positioning, so that the pedal can only be activated by the operator, intentionally.
-
Page 22: Use
Switching the Device On and Off Switching the Device On To switch the device on, set the switch button located on the left side of the device to position “I”, taking care not to press the foot pedal down during this stage. 4 symbols light up on the device (ref. U inside the cover) and progressively switch off. When this sequence is completed, the device is set to default parameters... - Page 23 FUNCTIONS (Ref. P front endpaper) Depending on the type of clinical application, it is possible to choose one of the 6 options available from the “function” list: • ENDO: dedicated to endodontic surgery, to Schneider’s membrane detachment and conventional endodontic treatments; • PERIO: dedicated to periodontal surgery and conventional scaling and periodontal treatments;...
- Page 24 CAUTION: The FLUSH function must WARNING: The “FLUSH” function be used after every patient treatment, does not substitute for the cleaning and before starting the cleaning and sterilization sterilization procedures described in the procedures. Failure to carry out flushing Cleaning and Sterilization manual. After of the handpiece tubing will lead to salt having carried out the “FLUSH”...
- Page 25 In the event that an irrigation capacity lower than 8 ml/min is required, use the “bone grafting kit” (accessory that can be ordered separately), inserting it between the irrigation set and the tube in silicon of the handpiece, making it pass through the peristaltic pump, and selecting 1 as the level of irrigation. WARNING: If the “bone grafting kit”...
- Page 26 SYMBOLS (Ref. S front endpaper) PIEZOSURGERY® GP is equipped with a diagnostic circuit that allows to detect function irrigation operating abnormalities and to view their implant type on the keyboard via their relative cortical symbol. pump/flush cancellous help user identify special malfunctioning part, four symbols are perio foreseen which are described in Chapter endo...
- Page 27 Excessive pressure on the inserts during their use can lead to their breakage. If an insert WARNING: Use of non-original breaks, check that none of its fragments Piezosurgery/Mectron inserts: this use remain in the treated part and, at the same entails finite damage to the handpiece time, apply effective suction to remove them. threading, thus compromising correct The patient must be instructed to breathe...
-
Page 28: Instructions For Use
CAUTION: Do not twist or rotate the foot pedal cable connector when inserting or removing it. Twisting can damage the connector. Instructions for Use After having connected all the accessories as described in Chapter 4.2 on page 11 , proceed as follows: Open the air inlet on the spike, before proceeding with the operation. - Page 29 Tighten insert using Piezosurgery/Mectron torque wrench. To correctly use the Piezosurgery/ Mectron torque wrench, operate as follows: Place the insert into the wrench as shown; Hold the central body of the handpiece firmly. CAUTION: The handpiece must not be grabbed by its terminal part and/ or cord, but only by its central body.
- Page 30 “Appropriate settings for the inserts on the PIEZOSURGERY® GP” or the illustrative leaflet of the purchased Piezosurgery/Mectron insert. NOTE: Check the level of physiological solution contained in the irrigation bag. Replace the irrigation bag with a new one before it is completely empty.
-
Page 31: Important Information On Inserts
It may • Use original Piezosurgery/Mectron happen that, while cutting the bone, inserts only. Use of non-original accidental contact of certain parts of inserts, in addition to voiding the... -
Page 32: Maintenance
The customer is entitled to deliver the old broken; device for disposal to the retailer supplying • Irrigation set: at the end of each the new equipment. Instructions on correct intervention; disposal are available from Mectron. Non-compliance with the previous points may • Peristaltic pump tube: after 8 sterilization produce a fine in accordance with the Waste cycles; Electrical and Electronic Equipment (WEEE) •... -
Page 33: Technical Specifications
TECHNICAL SPECIFICATIONS TECHNICAL SPECIFICATIONS Device compliant with Regulation (EU) Classe IIa 2017/745 Applied part type B (insert) Classification as per IEC/EN 60601-1 IP 20 (device) IP 22 (foot pedal - FS-05 model) According to the standard IEC 80601-2-60 Essential performance the device has no essential performance. 60sec. ON - 30sec. OFF with irrigation Device for intermittent operation 30sec. ON - 120sec. OFF without irrigation (ENDO, PERIO) Power supply voltage 100-240 V~ 50/60 Hz Max. power consumption 120 VA Fuses Type 5 x 20 mm, T 2AL, 250V Automatic scan Operating frequency From 24 KHz to 36 KHz ENDO PERIO SPECIAL Power settings CANCELLOUS CORTICAL IMPLANT Adjustable on the touch screen: ENDO / PERIO - 7 flow levels: from 0 to 6... -
Page 34: Guidance And Manufacturer's Declaration - Electromagnetic Emissions
PIEZOSURGERY® GP and the other devices information provided in this chapter. must be observed and checked to verify normal operation in the configuration in WARNING: Only use original which they will be used. Piezosurgery/Mectron accessories and spare parts. The use of cables and accessories not WARNING: Portable and mobile radio supplied by Piezosurgery/Mectron might communication appliances may affect proper negatively affect the EMC performances. -
Page 35: Accessible Parts Of The Casing
TECHNICAL SPECIFICATIONS 8.1.2 Accessible Parts of the Casing PIEZOSURGERY® GP is designed to operate in the electromagnetic environment specified below. The customer or the user of PIEZOSURGERY® GP should ensure that it is used in such an environment. Basic EMC Electromagnetic Phenomenon standard or test Immunity test levels Environment Guidance method The floor must be made of wood, concrete or ceramic Electrostatic... -
Page 36: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
8.1.3 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity 8.1.3.1 Power Connection A.C. Input PIEZOSURGERY® GP is designed to operate in the electromagnetic environment specified below. The customer or the user of PIEZOSURGERY® GP should ensure that it is used in such an environment. Basic EMC Electromagnetic Phenomenon standard or test... - Page 37 TECHNICAL SPECIFICATIONS d) If the frequency stepping skips over an ISM or m) r.m.s. , before modulation is applied. amateur band, as applicable, an additional test n) The ISM (industrial, scientific and medical) bands frequency shall be used in the ISM or amateur radio between 0,15 MHz and 80 MHz are 6,765 MHz to band. This applies to each ISM and amateur radio 6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz band within the specified frequency range.
-
Page 38: Points Of Contact With The Patient
8.1.3.2 Points of Contact with the Patient PIEZOSURGERY® GP is designed to operate in the electromagnetic environment specified below. The customer or the user of PIEZOSURGERY® GP should ensure that it is used in such an environment. Basic EMC Electromagnetic Phenomenon standard or test Immunity test levels Environment Guidance method The floor must be made of wood, concrete or ceramic Electrostatic ±8 kV on contact tiles. If the floor is covered... -
Page 39: Parts Accessible To The Input / Output Signals
TECHNICAL SPECIFICATIONS 8.1.3.3 Parts Accessible to the Input / Output Signals PIEZOSURGERY® GP is designed to operate in the electromagnetic environment specified below. The customer or the user of PIEZOSURGERY® GP should ensure that it is used in such an environment. Basic EMC Electromagnetic Phenomenon standard or test Immunity test levels Environment Guidance method The floor must be made of wood, concrete or ceramic Electrostatic... -
Page 40: Specifications Of The Tests For The Immunity Of The Accessible Parts Of The Casing To The Wireless Rf Communications Device
8.1.4 Specifications of the tests for the Immunity of the Accessible Parts of the Casing to the Wireless RF Communications Device PIEZOSURGERY® GP is designed to operate in an electromagnetic environment in which radiated RF disturbances are under control. The customer or the user of PIEZOSURGERY® GP can help prevent electromagnetic interference by ensuring a minimum distance between the mobile and portable RF (transmitters) communication devices and PIEZOSURGERY® GP, as recommended, in relation to the maximum output power of radiocommunications equipment. -
Page 41: Troubleshooting
TROUBLESHOOTING NOTE: If necessary to achieve the WARNING: Portable RF communication IMMUNITY TEST LEVEL, the distance equipment (including peripheral devices as between the transmitting antenna and the antenna cables and external antennas) must PIEZOSURGERY® GP may be reduced to 1 not be used closer than 30 cm (12 inches) to m. The 1 m test distance is permitted by IEC any part of the device PIEZOSURGERY®... - Page 42 Symbol on the Keyboard Possible Cause Solution Check that there are no Peristaltic pump malfunction. impediments to pump rotation. Correctly reposition the Tubing not correctly silicon tubing inside the positioned in the peristaltic pump (see Chapter 4.2 on pump. page 11 The device has been turned Turn device off and wait 5 off and on again without seconds before turning it on...
-
Page 43: Troubleshooting Quick Guide
Unscrew the insert and screw The insert is not properly it back correctly by using the tightened into the Mectron torque wrench. handpiece. (Refer to Chapter 5.3 on When operating, page 20... - Page 44 (Refer to Chapter 4.2 on handpiece. page 11 Unscrew the insert and screw it back correctly by using the The insert is not correctly Mectron torque wrench. tightened on the handpiece (Refer to Chapter 5.3 on Poor performance page 20 Broken, worn-out or Replace the insert with a new deformed insert.
-
Page 45: Fuses Replacement
TROUBLESHOOTING 9.3 Fuses Replacement WARNING: Switch the device off. Always turn the device off by means of the main switch and disconnect it from the electrical power socket before proceeding. Apply leverage with a flat screwdriver, inserting its tip in the seat of the fuse- holder drawer located under the power supply socket; Pull out the fuse-holder drawer;... -
Page 46: Customer Service - Returns And/Or Repairs
9.4 Customer Service - Returns and/or Repairs If you need technical assistance regarding the Please provide the following information: use, or you encounter a problem that requires • Data of the owner with telephone servicing or repair, contact PIEZOSURGERY number; Inc. Customer Service at (1.888.87-PIEZO). • Product name; Returning products for any reason, requires •... -
Page 47: Warranty
If upon examination by PIEZOSURGERY Inc. ® The manufacturer, Mectron S.p.A., warrants service personnel it is determined that the to the first original purchaser (customer) that malfunction is caused by abnormal wear and their products have been tested, inspected tear or by damage caused by misuse, abuse, and shipped in proper working order. - Page 48 Manufacturer: Distributed in U.S. by: Mectron S.p.A. Via Loreto 15/A 16042 Carasco (Ge) Italy Tel. +39 0185 35361 850 Michigan Avenue Fax +39 0185 351374 Columbus, Ohio 43215 www.mectron.com Phone 614.459.4922 e-mail: mectron@mectron.com Fax 614.459.4981 www.piezosurgery.us e-mail: info@piezosurgery.us Reseller - Rivenditore - Wiederverkäufer - Revendeur - Revendedor...
















Need help?
Do you have a question about the PIEZOSURGERY GP and is the answer not in the manual?
Questions and answers