Subscribe to Our Youtube Channel
Summary of Contents for Biegler RESPIFIT S
- Page 1 Instructions for use Patients RESPIFIT S Inspiratory Muscle Training Device Edition 2021-05-18 | 40-246-03 Sales Manufacturer...
- Page 2 These instructions for use are aimed at patients who use the RESPIFIT S. More information on the settings and functions is available for clinical users such as physiotherapists, care staff and doctors in the RESPIFIT S (clinic) instructions for use.
-
Page 3: Table Of Contents
ANDHELD PATIENT MODULE ..........................11 ATAKEY ................12 ONNECTING THE DEVICE AND ACCESSORIES ....................12 ETTING UP BEFORE TRAINING TRAINING WITH THE RESPIFIT S .................... 13 ......................13 HE STRENGTH EXERCISE ....................... 16 HE ENDURANCE EXERCISE ........................19 ESULTS DISPLAY ........................ -
Page 4: Description
The RESPIFIT S can be used to set up training programmes to increase both strength and endurance of the respiratory muscles. Integrated functions can also determine the capacity of the inspiratory muscles, i.e. -
Page 5: Indication
1.5 Scope of delivery Number Description Catalogue number LR1003004 RESPIFIT S device from SN: 402005 FK1003008 RESPIFIT S instructions for use clinic and patient FK1003009 Handheld patient module IB1002001 Datakey AG6110050 Power supply unit Mean Well Type... -
Page 6: Accessories
Power supply unit Mean Well Type GEM06I05 CJ1003004 2 GENERAL SAFETY INSTRUCTIONS • RESPIFIT S may only be used under the direction of the treating physician / therapist. • RESPIFIT S (mouthpiece of the handheld patient module) may only be used if the mucous membrane is intact. - Page 7 RESPIFIT S • RESPIFIT S may only be used in areas in which the electrical installations comply with the standards and regulations in force. • This device is subject to specific preventive measures with regard to electromagnetic compatibility (EMC) and must therefore be set up and commissioned in accordance with the EMC instructions contained in Section 12 of these instructions for use.
-
Page 8: Initial Operation And Use
• If the device is plugged in without the datakey inserted, the display indicates “DATAKEY “ • Measurements using RESPIFIT S serve only as a basis for training settings and do not constitute a complete determination of pulmonary function. For diagnosis and monitoring, measurements must be carried out by the treating physician / therapist, independently of the RESPIFIT S. -
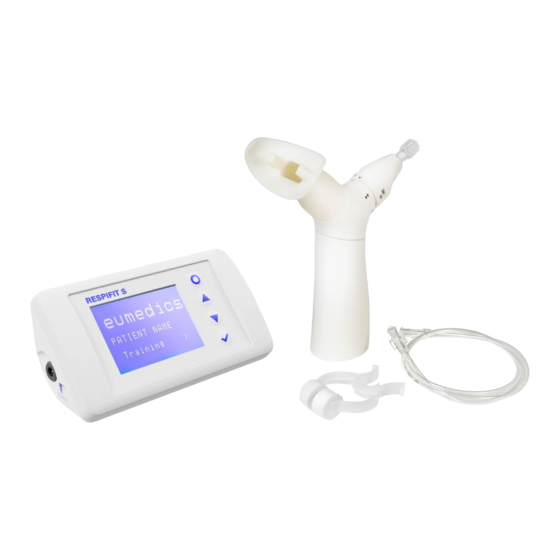
Page 9: Handheld Patient Module
RESPIFIT S Figure 1: RESPIFIT S in packaging 3.1 Handheld patient module The handheld patient module must only be used repeatedly by a single patient (refer to cleaning instructions in Section 6). Important: The maximum service life of the handheld patient module is three months. - Page 10 RESPIFIT S Figure 2: Resistance RES in the aperture diameters A, B or C Once the appropriate aperture has been selected, the adjustment mechanism is pushed down and locked and is therefore inaccessible to the patient. Thereafter the adjustment mechanism can no longer be released.
-
Page 11: Datakey
Figure 4: Connecting the handheld patient module 3.2 Datakey Before the patient starts therapy with the RESPIFIT S, the patient- specific settings must be determined under the direction of the treating physician / therapist and stored on the datakey. -
Page 12: Connecting The Device And Accessories
RESPIFIT S 3.3 Connecting the device and accessories Insert the connector plug of the connection cable into the labelled socket of the device and connect the power supply unit to the mains power. GEM06I05 Figure 6: Connecting the power supply unit to the device Connect the connection tube to the handheld patient module and the device with Luer connectors. -
Page 13: Training With The Respifit S
RESPIFIT S 4 TRAINING WITH THE RESPIFIT S IMPORTANT RESPIFIT S may only be used under the direction of the treating physician / therapist. Prior to training, commission the device and accessories as described in Section 3. After switching on the device (plugging in the mains plug) with the datakey inserted, a welcome screen appears. - Page 14 RESPIFIT S strength value is not achieved at all or not achieved in one breath (coughing, etc). It is also deemed to be a failed attempt if the dumbbell is not held above the preset minimum strength value for one full second. If there are more defective breaths than are permitted, the exercise block is closed and stored on the datakey as a failed training session.
- Page 15 RESPIFIT S The training screen for the strength exercise is structured as follows: Figure 12: Strength exercise training screen The strength bar shows how high the dumbbell must be lifted for each exercise. The dumbbell must be held above the preset minimum strength value for one second.
-
Page 16: The Endurance Exercise
RESPIFIT S The strength training is concluded either once the required number of individual exercises has been completed or the number of permitted failed attempts has been exceeded. A separate screen indicates whether the training (exercise block) has been successfully completed or not. - Page 17 RESPIFIT S For the endurance exercise, the following parameters must be set in advance by the treating physician / therapist: • Number of endurance exercises (per exercise block) • Number of failed attempts (per exercise block) • Pause time (between the individual exercises) •...
- Page 18 RESPIFIT S and the ground, depicted symbolically on the screen, represent the boundaries within which the balloon must be moved. During the exercise, the seconds indicator continuously counts down from 60 seconds and the time bar diminishes accordingly. The number of individual exercises is displayed next to the "exercise"...
-
Page 19: Results Display
RESPIFIT S Results display The results of the exercises can be displayed by pressing on the start screen. Figure 24: Results selection on the device The results of the exercises performed are displayed along with any stops during the training session. -
Page 20: Cleaning
RESPIFIT S CLEANING IMPORTANT Before cleaning, the device must always be disconnected from the mains power supply. The device may only be cleaned using a soft cloth with water-soluble, non-abrasive cleaning agents or special cleaning agents for plastics. In all instances of cleaning, never allow any liquid to get into the device or into the transparent measuring lead (tube) of the handheld module. - Page 21 RESPIFIT S disinfection agent must be followed exclusively. After the disinfection procedure, let the handheld patient module dry. Use of the disinfection agent may cause slight discolouration of the plastic. However, the function of the handheld patient module will not be affected by this.
-
Page 22: Maintenance
RESPIFIT S 6 MAINTENANCE The inspiratory muscle training device RESPIFIT S is designed as a low- maintenance device. For long-term maintenance of quality and functional safety, please observe the following points: The handheld patient module must only be used repeatedly by a single patient for reasons of hygiene. -
Page 23: Returning Devices Or Accessories
• The power supply unit and cables must be visually inspected (only Biegler original parts are permitted, see section 1.6). • Housing and patient leakage currents must be checked. The results of the periodic inspection must be documented, along with the date, the inspecting agency and the device number. -
Page 24: Manufacturer Liability
RESPIFIT S 9 MANUFACTURER LIABILITY The manufacturer and the supplier of the device / accessory reject any liability if • the device is not used in accordance with the instructions for use. • the patient or doctor / therapist is not sufficiently informed about the way the device functions as per the instructions for use and the safety instructions. - Page 25 RESPIFIT S The warranty will also be void if original Biegler materials were not used as replacement parts, or measures for repair were undertaken by persons not authorized by the manufacturer or supplier. If the manufacturer is required to meet a warranty claim in accordance with these terms, the customer shall bear the costs and risks of transport of the device from and to the place of use.
-
Page 26: Electromagnetic Compliance
RESPIFIT S 11 ELECTROMAGNETIC COMPLIANCE 11.1 Emissions Test Limits Conducted emissions CISPR 11, Group 1, Class B Radiated emissions CISPR 11, Group 1, Class B Harmonic current emissions IEC 61000-3-2, Class A (IEC 61000-3-2) Voltage changes and flicker IEC 61000-3-3, complies with requirements (IEC 61000-3-3) 11.2 Immunity test level... -
Page 27: Symbols
RESPIFIT S Power cables: 2 kV; 100 kHz repetition Electrical fast frequency transients/bursts Signal lines: 1 kV; 100 kHz repetition (IEC 61000-4-4) frequency Surges L-PE and N- PE: 2kV (IEC 61000-4-5) L-N: 1kV HF conducted 0.15–80 MHz; 1kHz AM 80%; 3 Vrms,... -
Page 28: Operating And Storage Conditions
(refer to cleaning instructions) Batch number Catalogue number 13 OPERATING AND STORAGE CONDITIONS The RESPIFIT S is intended for use in the following facilities: • Medical establishments, incl. on-site examination rooms, general practices and therapy rooms. • Home use Permissible environmental conditions for the device and accessories:... -
Page 29: Technical Data
Dimensions of handheld module: W x D x H 60 x 140 x 162 mm Pressure measurement Measuring range: -180 mbar Resolution: 1 mbar Precision: +/- 4 % RESPIFIT S product life: 5 years Product life of the handheld patient 3 months module:... -
Page 30: Manufacturer And Sales
Fax. +43 1 577 35 60 33 office@biegler.com office@eumedics.at www.biegler.com www.eumedics.at 16 MANUFACTURER’S DECLARATION The inspiratory muscle training device RESPIFIT S is a medical device as defined by Directive 93/42/EEC. This is documented through the CE mark. Notified Body: TÜV SÜD Product Service GmbH, Approval number... - Page 31 RESPIFIT S NOTES...







Need help?
Do you have a question about the RESPIFIT S and is the answer not in the manual?
Questions and answers