Subscribe to Our Youtube Channel
Summary of Contents for Biegler autopress
- Page 1 Instructions for use automatic pressure controller Version 2018-07-31 40-202-01...
- Page 2 IMPORTANT These directions are essential for operating the device. They must therefore be kept in a suitable place near the device, and should be kept with the device if it is given to other users. This manual is valid for devices with serial number 381000 or higher. For proper and safe use of this device, it is essential that the following warnings and safety instructions, as well as the operating instructions, are read and carefully observed by all users before first using the device.
-
Page 3: Table Of Contents
TABLE OF CONTENTS Warnings and Safety Instructions ............. 4 Description ....................6 2.1 General Discription ..................6 2.2 Scope of Delivery ..................6 Initial Operation ................... 7 Maintenance ....................9 Cleaning and Disinfection ................ -
Page 4: Warnings And Safety Instructions
Only pressure infusion bags specified by BIEGLER or approved by BIEGLER for use with this device may be used in conjunction with the autopress. The device must only be used in areas in which the electrical installations are in accordance with the standards and regulations in force. - Page 5 The pressure infusion bags must be securely fastened at least 20 cm above the patient to prevent air embolism. Prevent the connecting hose from kinking. Always position the autopress in such a way that it is easy to operate without obstacles. No other devices or infusion stands shall be positioned shortly before.
-
Page 6: Description
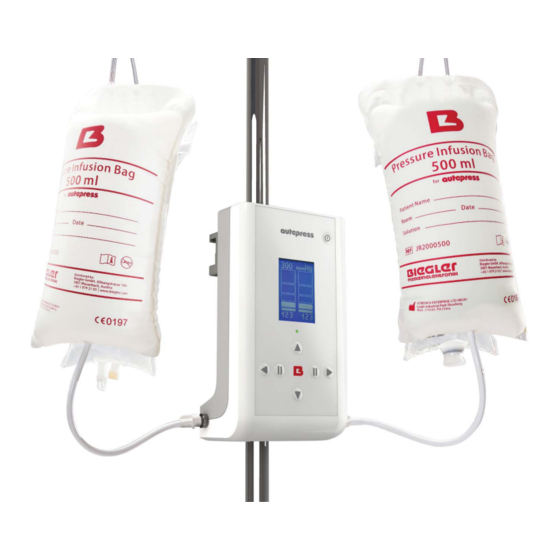
Description Article number autopress LG4000003 The automatic pressure controller autopress is used when liquids are to be supplied under constant pressure. The autopress can be used in combination with all BIEGLER Blood- and Infusionwarmers or used as standalone device. Only the following single-use pressure infusion bags can be used:... -
Page 7: Initial Operation
The condition of the patient has to be monitored during the application. 1. Fix the autopress firmly on a stand using the clamps at the back. Only use infusion stands, tripods or equipment rails that are sufficiently stable. - Page 8 Operation: clamp ON / OFF set pressure ratio actual to set pressure actual pressure Power supplied Standby-LED increase vent left pressure channel vent right channel activate right activate left channel channel decrease pressure Figure 2 - Operation, Display...
-
Page 9: Maintenance
4. MAINTENANCE The autopress was largely designed as a maintenance-free device. For long-term maintenance of quality and functional safety, we would like to ask you to observe the following points: Always keep the device clean (see the "Cleaning and disinfection" section). -
Page 10: Periodic Inspections
The periodic technical safety inspections (according to the local standards in force - e.g. in Austria, EN 62353) on the autopress must be carried out at least every 12 months, by persons authorized to carry out such inspections based on their training, knowledge and practical experience. -
Page 11: Warranty Conditions
The warranty will also be void if original BIEGLER materials were not used as replacement parts, or measures for repair were undertaken by persons not authorized by the manufacturer or supplier. -
Page 12: Return Of Devices
9. RETURN OF DEVICES Devices must be carefully cleaned and disinfected before being placed in the original packaging for returning. If the original packaging is no longer available, the product has to be suitably packaged for the method of dispatch. 10. -
Page 13: Symbols
11. SYMBOLS compliance with Directive Serial number 93/42/EEC Consult instructions for use Catalogue number Attention, consult Manufacturer accompanying documents Defibrillation-proof type CF manufacturing date applied part On / OFF AC voltage activate right channel increase pressure activate left channel decrease pressure Degree of protection pause button –... -
Page 14: Operating And Storage Conditions
13. EMC TABLES Table 1 Guidelines and manufacturer's declaration – electromagnetic emissions The autopress is intended for use in the environment specified below. The customer or the user of the autopress should ensure that it is used in such an environment. Interference... - Page 15 Table 2 Guidelines and manufacturer's declaration – electromagnetic interference resistance The autopress is intended for use in the electro-magnetic environment specified below. The customer or the user of the autopress should assure that it is used in such an environment.
- Page 16 Table 4 Guidelines and manufacturer's declaration – electromagnetic interference resistance The autopress is intended for use in the electro-magnetic environment specified below. The customer or the user of the autopress should ensure that it is used in such an environment.
- Page 17 Recommended safety distances between portable and mobile RF telecommunications devices and the autopress The autopress is intended for use in an electromagnetic environment in which radiated RF disturbance variables are controlled. The customer or user of the autopress can help prevent electromagnetic interference...
-
Page 18: Technical Data
14. TECHNICAL DATA Device: automatic pressure controller Type designation: autopress Operating voltage: 100 – 240 Vac / 50 – 60 Hz Power consumption: max. 36 VA Supply type: mains operation Protection class: Degree of protection against electric shock Type CF, defibrillationproof... -
Page 19: Manufacturer's Declaration
15. MANUFACTURER’S DECLARATION The autopress is a medical product as defined by Directive 93/42/EEC. This is documented through the CE mark. Notified Body: TÜV SÜD Product Service, Approval Number CE0123 16. MANUFACTURER Biegler GmbH Allhangstrasse 18a 3001 Mauerbach AUSTRIA Tel. +43 1 979 21 05 Fax +43 1 979 21 05 16 office@biegler.com...







Need help?
Do you have a question about the autopress and is the answer not in the manual?
Questions and answers