Subscribe to Our Youtube Channel
Summary of Contents for Biegler Autopress
- Page 1 Instructions for use automatic pressure controller Version 2020-08-27 40-202-02...
- Page 2 IMPORTANT These directions are essential for operating the device. They must therefore be kept in a suitable place near the device, and should be kept with the device if it is given to other users. For proper and safe use of this device, it is essential that the following warnings and safety instructions, as well as the operating instructions, are read and carefully observed by all users before first using the device.
-
Page 3: Table Of Contents
Table of Contents 1. Warnings and Safety Instructions ............4 2. Description....................6 General Description................6 Scope of Delivery ................6 Intended use ..................7 Indication..................7 Contraindication ................7 3. Initial Operation ..................8 4. Maintenance..................10 5. Cleaning and Disinfection..............10 6. -
Page 4: Warnings And Safety Instructions
The pressure infusion bags must be securely fastened at least 20 cm above the patient to prevent air embolism. Prevent the connecting hose from kinking. Always position the autopress in such a way that it is easy to operate without obstacles. No other devices or infusion stands shall be positioned shortly before. - Page 5 Make sure, that mains plug of the autopress is easily reachable to the operator. (The mains plug is used to disconnect the device from mains) The automatic pressure controller autopress is a class I ME equipment and therefore only intended to be connected to supply mains with protective earth.
-
Page 6: Description
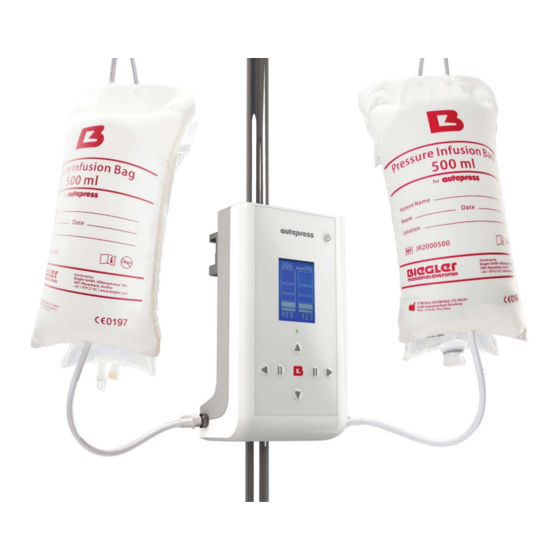
Description Article number autopress LG4000003 The automatic pressure controller autopress is used when liquids are to be supplied under constant pressure. The autopress can be used in combination with all BIEGLER Blood- and Infusionwarmers or used as standalone device. The following single-use pressure infusion bags can be used:... -
Page 7: Intended Use
Intended use The automatic pressure controller autopress is designed for use in IV infusion therapy and for applications where IV infusions or irrigations are to be supplied under constant pressure. Indication The automatic pressure controller autopress can be used wherever liquids are to be administered under pressure. -
Page 8: Initial Operation
The condition of the patient has to be monitored during the application. 1. Fix the autopress firmly on a stand using the clamps at the back. Only use infusion stands, tripods or equipment rails that are sufficiently stable. - Page 9 Operation: clamp ON / OFF set pressure ratio actual to set pressure actual pressure Power supplied Standby-LED increase vent left pressure channel vent right channel activate right activate left channel channel decrease pressure Figure 2 - Operation, Display...
-
Page 10: Maintenance
The periodic technical safety inspections (according to the local standards in force - e.g. in Austria, EN 62353) on the autopress must be carried out at least every 12 months, by persons authorized to carry out such inspections based on their training, knowledge and practical experience. -
Page 11: Manufacturer Liability
The results of the periodic inspection must be documented, along with the date, the inspecting agency and the device number. Important: If a malfunction is discovered during the periodic inspection, suitable warning signs should be attached to the device to ensure that it is not used before the required service and repair work has been carried out. -
Page 12: Warranty Conditions
The warranty will also be void if original BIEGLER materials were not used as replacement parts, or measures for repair were undertaken by persons not authorized by the manufacturer or supplier. -
Page 13: Return Of Devices
Return of Devices Devices must be carefully cleaned and disinfected before being placed in the original packaging for returning. If the original packaging is no longer available, the product has to be suitably packaged for the method of dispatch. 10. Disposal Dispose of the device or its accessories in accordance with local regulations. -
Page 14: Symbols
11. Symbols Compliance with Directive Serial number 93/42/EEC Manufacturer Consult instructions for use Manufacturing date Attention Defibrillation-proof type CF applied part On / OFF AC voltage Increase pressure Activate right channel Activate left channel Decrease pressure Degree of protection Pause button – IPX4 against ingress of water - vent the channel... -
Page 15: Operating And Storage Conditions
12. Operating and Storage Conditions Permissible environmental conditions for transporting and storing the autopress and accessories: Transport and storage Operating Temperature 10 – 40 °C 10 – 30 °C Relative humidity 30 – 75 % 30 – 75 % Ambient pressure 700 –... - Page 16 385 MHz; Pulse Modulation: 18 Hz; 27 V/m 450 MHz, Pulse Modulation: 18 Hz: 1 kHz sine; 28 V/m 710, 745, 780 MHz; Pulse Modulation: 217 Hz; 9 V/m Proximity fields form RF wireless 810, 870, 930 MHz; communications Pulse Modulation: 18 Hz; 28 V/m equipment (IEC 61000-4-3) 1720, 1845, 1970 MHz;...
-
Page 17: Technical Data
14. Technical Data Device: automatic pressure controller Type designation: autopress Operating voltage: 100 – 240 Vac / 50 – 60 Hz Power consumption: max. 36 VA Supply type: mains operation Protection class: Degree of protection against electric shock Type CF, defibrillationproof... -
Page 18: Manufacturer's Declaration
15. Manufacturer’s Declaration The autopress is a medical product as defined by Directive 93/42/EEC. This is documented through the CE mark. Notified Body: TÜV SÜD Product Service GmbH, Approval Number CE0123 16. Manufacturer Biegler GmbH Allhangstrasse 18a 3001 Mauerbach Austria Tel.







Need help?
Do you have a question about the Autopress and is the answer not in the manual?
Questions and answers
Is there documentation that indicates if the Biegler Autopress can be used for Urology cases?
The Biegler Autopress is designed for use in IV infusion therapy and for supplying liquids under constant pressure. It is not specifically indicated for urology cases. Therefore, based on the provided information, it cannot be confirmed that it is suitable for urology cases.
This answer is automatically generated