Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for AKRUS ak 5010 MBS
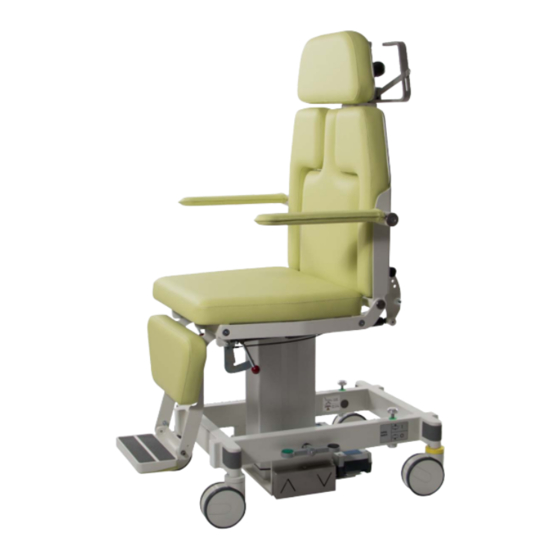
- Page 1 Mobile Mammography Chair ak 5010 MBS User Manual...
-
Page 2: Table Of Contents
Index GENERAL ..............................3 ..............................3 OPYRIGHT ............................... 3 ISCLAIMER ......................3 PPLICABLE RULES AND REGULATIONS ......................... 3 ARNING AND CAUTIONING SIGNS ....................... 4 SER INSTRUCTIONS FOR SAFE OPERATION ..........................4 RODUCT DENOMINATION USEFUL LIFE AND WARRANTY CONDITIONS .................... 5 SCOPE OF DELIVERY .......................... -
Page 3: General
General To safely operate and control the ak 5010 MBS please carefully read this user manual before operating. Please pay attention to all cautionary statements pertaining to the safe operation of the ak 5010 MBS. Please keep the manual on file at all times while the product is in service. -
Page 4: User Instructions For Safe Operation
User instructions for safe operation Before using this medical device, please carefully read and observe the safety instructions and recommendations listed in this manual Caution The device may only be installed, operated, used and maintained Risk of operat- by persons who have been properly trained or who have the re- ing errors quired knowledge and experience to do so. -
Page 5: Useful Life And Warranty Conditions
The ak 5010 MBS is intended for use as an examination chair. Any other use of the product is not permitted The ak 5010 MBS is designed for a maximum patient weight of 135 kg (330 lbs) A 250 kg (550 lbs) version is available upon request. -
Page 6: Intended Use
4.1 Intended use The medical has been designed to support / position patients for diagnostic or therapeutic pro- cedures. The standards covering operating tables (EN 60601-2-46) are not applicable for this product. The essential performance is the ability to position a patient in a controlled manner by means of control elements, within the scope of movement possibilities. -
Page 7: Battery Re Charging Intervals
Battery re charging intervals The battery should be re charged every 2-3 days, in case of heavy duty usage (> 30 full cycle move- ments/day) re charge daily. The re charger protects the battery from damage by overloading, so the battery may be re charged overnight or during the week end. -
Page 8: Battery Holder At The Ak 5010 Mbs Chair
Position of battery holder Electric lift (101-016 / 101-017) and foot switch (277.012003) The ak 5010 MBS is powered by a low voltage lift The Up – Down movement are con- trolled... -
Page 9: Control Lever For Heavy Duty Castors
Control lever for heavy duty castors The chair features 4 heavy-duty cas- tors and two foot levers on either side of the lower frame. The three settings below are available All wheels locked (kick on black arm) All wheels unlocked and swiveling (set arms horizontal) All wheel unlocked, one wheel set for steering (kick on green arm) Foot operated control lever in different settings one wheel locked for direc-... -
Page 10: Warning And Cautioning Signs On The Chair
Warning and cautioning signs on the chair option 277g001e_ak5010_3rd ed_MDR_REV 7.0.docx26.05.2021 Page 10 of 23... - Page 11 Manufacturer Date of manufacturing EU- symbol of confornity Anwendungsteil vom Typ B gemäß IEC 60601-1 Use only indoors. do not dispose of through general household waste Order no/ part no Serial no Danger to patient, operator or device North America – symbol of confornity Identifier Device is a medical...
- Page 12 Weight of chair Max patient weight (135kg) option option Weight of chair Max patient weight (250kg) Prohibition sign “DO NOT PLACE ANY LOAD“ DIN 4844-2001 Any load > 200N is not permissible 277g001e_ak5010_3rd ed_MDR_REV 7.0.docx26.05.2021 Page 12 of 23...
-
Page 13: Operation Of Chair
Operation of chair Duty cycle (continuous operation) of electrical motors The electrical motors are designed for a duty cycle of approximately 6 minutes at full load. Continuous operations exceeding that time period may cause overheat conditions and permanent damage to the transformer. -
Page 14: Adjustment Of Armrests
8.2.2 Adjustment of armrests Both arm rests fold away upwards and can be placed parallel to the back rest. 8.2.3 Adjustment of back rest The back rest is infinitely ad- justable from vertical to fully horizontal. Pressing one of the two spring loaded levers behind the head rest (arrow) unlocks the pneumatic gas spring and the back rest will... -
Page 15: Adjustment Of Back Rest Segments
8.2.5 Adjustment of back rest segments The back rest offers two in- dividually adjustable seg- ments, allowing to position patients laterally (park bench). To make any adjust- ment, secure segment with one hand, then pull on the peg (arrow). To lock seg- ment in position, just let the peg latch into the desired lock hole. -
Page 16: Trendelenburg (277.025010)
Propanol=35% // Ethanol=25% This would equal for example Terralin Liquid offered by. Schülke&Mayr. o Do not attempt to sterilize the ak 5010 MBS o Clean only the external surfaces of the equipment using a damp cloth. In case of heavy stains do not use abrasive or aggressive materials other than common cleansers or detergents. -
Page 17: Disposal Of Device
12 Disposal of device The battery and all electrical components must be disposed of in accordance with national applicable laws and regulations. All other components are household waste. 13 Technical Data Technical Data Value Unit Dimensions and weight Length wheel base (backrest up) Width wheel base Width chair incl. -
Page 18: Environmental Conditions
16 CE Conformity We declare the compliance of the device with the requirements of the Council Directive MDR 2017/745 about Medical Devices class I. 17 Manufacturer AKRUS GmbH & Co KG Otto-Hahn-Straße 3 D-25337 ELMSHORN int. +49 (0) 4121 791930 Email: info@akrus.de... -
Page 19: Electromagnetic Compatibility Emc
• Portable and mobile HF communications equipment may cause an effect to the ak 5010 MBS CHAIR. Use no cell phones and no other devices that do not comply with the EMC class B CISPR 11 near the instrument. •... - Page 20 The ak 5010 MBS chair is intended for use in the electromagnetic environment specified below. The customer or the user of that ak 5010 MBS chair should ensure that it is used in such an environment. Electromagnetic environment – guide lines...
- Page 21 Guidance and manufacturer's declaration - electromagnetic immunity The ak 5010 MBS chair is intended for use in the electromagnetic environment specified below. The customer or the user of the ak 5010 MBS chair should ensure that it is used in such an environment. IEC 60601...
- Page 22 If the measured field strength in the location at which the ak 5010 MBS chair is used, ex- ceeds the compliance level, the patient lying S60 should be observed to demonstrate the intended function.
- Page 23 5010 MBS chair The ak 5010 MBS chair is intended for use in an electromagnetic environment in which the RF disturbances are con- trolled. The customer or the user of the ak 5010 MBS chair this can help prevent electromagnetic interference by...








Need help?
Do you have a question about the ak 5010 MBS and is the answer not in the manual?
Questions and answers