Vitalograph In2itive e-Diary Instructions For Use Manual
Hide thumbs
Also See for In2itive e-Diary:
- Instructions for use manual (28 pages) ,
- Instructions for use manual (56 pages)
Summary of Contents for Vitalograph In2itive e-Diary
- Page 1 In2itive e-Diary MODEL 2120 Instructions for Use © Copyright Vitalograph 2020 Current Edition (Issue 2, 25-Aug-2020) Cat. No. 09180...
- Page 2 Tel: +353 65 6864111 Technical Support Email: technical.support@vitalograph.ie Tel: +353 65 6864111 Email: technical.support@vitalograph.ie Vitalograph GmbH Rellinger Straße 64a Vitalograph Ltd, Hong Kong/China D-20257 Hamburg P.O. Box 812 Germany Shatin Central Post Office Tel: +49 40 547391-40 Hong Kong Fax: +49 40 547391-40 E-mail: sales@vitalograph.cn...
-
Page 3: Table Of Contents
Customer Service ..............21 Consumables and Accessories .........22 Disposal ................22 10. Explanation of Symbols ............22 11. Description of the Vitalograph 2120 In2itive e-Diary ..23 11.1. Indications for Use ..........24 12. Technical Specification ............25 13. Contraindications, Warnings, Precautions and Adverse Reactions ..............28 14. -
Page 4: Main Components
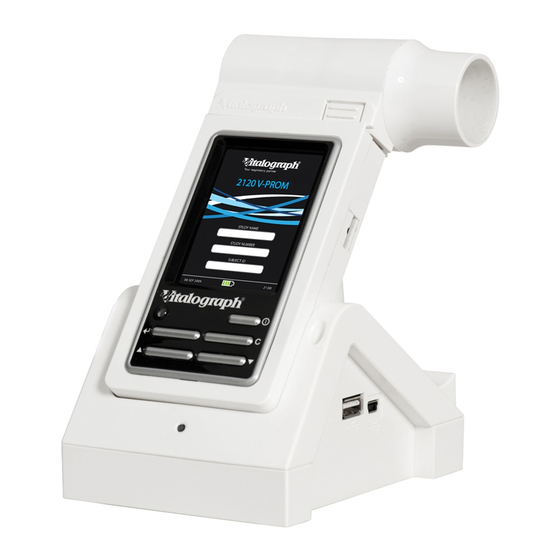
Flowhead Flowhead Mouthpiece LCD/Touch Panel Display Either a PC or a Vitalograph COMPACT Expert medical workstation is required to run the Spirotrac application and to interface the device with the Vitalograph web portal. Note: PC/COMPACT not included with the device. -
Page 5: Features
• Fleisch type pneumotachograph flowhead 2. Set Up and Return The Vitalograph 2120 In2itive e-Diary is a clinical trial device and should be set up for a subject by a healthcare professional prior to use. The subject then takes the device home. Study site instructions include specific detail on device set up, based on the requirements for the device being used in the study. - Page 6 On the COMPACT/PC select ‘Connect’ to enter remote mode on the device, as in Figure 2. Figure 2: Connecting the device to the Vitalograph web portal h. At first time set up, the user is informed that the device is blank.
-
Page 7: Downloading Data After The Device Is Returned To The Site By The Subject
3. Downloading data after the device is returned to the site by the subject: a. Power on the COMPACT/PC and log in to the Vitalograph web portal using the username and password combination provided by email. b. Turn on the device. When prompted use the USB cable provided to connect the device to a USB port on the COMPACT/PC running Spirotrac. -
Page 8: Operating Instructions
3. Operating Instructions The Vitalograph 2120 In2itive e-Diary is a clinical trial device and should be set up for a subject by a healthcare professional prior to use. Any configuration required is completed prior to issuing the device to the subject, as per Section 2. -
Page 9: Main Menu
In2itive e-Diary - Instructions for Use 09180 Issue 2 Figure 3: The device alarms when it is time to complete a session. If the subject misses the alarm, they may turn on the device using the On/Off power button on the side of the device and complete the session so long as it is within the specified time window. -
Page 10: Subject Operating Instructions
A Global Leader in Respiratory Solutions Begin Session: Follow the on-screen instructions to complete a session, starting with the relevant questionnaire and then the flow test. Training: Includes information and videos on time windows, and performing a blow including blow faster, blow longer, do not cough, blow not consistent, accuracy, reproducibility and effort quality. - Page 11 In2itive e-Diary - Instructions for Use 09180 Issue 2 Example script for performing a good blow: a. Sit down in a chair. b. Keep back straight. c. Keep shoulders back. d. Feet are flat on the ground. e. When the ‘blow now’ animated icon appears on screen the device is ready for a test.
- Page 12 A Global Leader in Respiratory Solutions Figure 6: Example Completed Test Screen 3. The results of the test session are displayed when all blows for the session have been performed. Figure 7 shows results screens for acceptable and unacceptable quality test sessions: Figure 7: Acceptable Vs Unacceptable Test Quality Page of 40...
-
Page 13: Calibration Verification
1 to 2 seconds. The device will auto power down after 5 minutes of inactivity. 5. Data is automatically sent to Vitalograph study servers once the session has completed. Figure 8: Test Session Complete 3.2. - Page 14 A Global Leader in Respiratory Solutions Figure 9: Calibration verification screen 1. Connect the device flowhead to the syringe. 2. Enter details of the Manual 3L syringe, make a note of the temperature. Press Continue. 3. Complete 3 strokes as per onscreen instructions. The volume in (L) displays for each stroke.
-
Page 15: Cybersecurity Considerations
OS and is the only user interface to the device. The OS has a configured firewall to protect against any unauthorised access over the USB port, only Vitalograph PC based applications can access the device over secure USB connection. No further controls are required by users to secure the device. -
Page 16: Power Management
If the device becomes inoperable either by fault or compromise, the device should be returned to Vitalograph where the data can be restored to the fixed/ replacement device. Note: The device should not be used if the tamper evident seal is broken. -
Page 17: Battery Pack
In2itive e-Diary - Instructions for Use 09180 Issue 2 When powered from a power supply or USB, the plug icon displays on the status bar, next to the battery icon. The device emits an alarm which turns on the device when it is time to complete a session. -
Page 18: Battery Power Indications
A Global Leader in Respiratory Solutions 4.2. Battery Power Indications Battery is fully charged: a white ‘Battery Full’ icon displays on the status bar at the top of the screen. Disconnect the device from an external power source when it is fully charged. Battery is at less than 20% capacity: an orange ‘Battery Low’... -
Page 19: Inspection Of The Vitalograph 2120 In2Itive E-Diary
The Vitalograph 2120 In2itive e-Diary is not designated as a sterile device. It is a non-critical, single patient use device. One cleaning cycle of the case/flowhead should be performed weekly. -
Page 20: Fault Finding Guide
A Global Leader in Respiratory Solutions 6. Fault Finding Guide Problem Fault • False readings suspected Symptoms: Possible • Flowhead blocked or electronics Solution: (In failure – contact support*/study site probable order) administrator. Problem Fault • Battery red icon displayed, device powers Symptoms: down immediately. -
Page 21: Software Check
7. Customer Service This device has no serviceable parts. The device must be returned to the manufacturer, Vitalograph, at the end of the Study. Repairs should only be carried out by the manufacturer. Contact the manufacturer for assistance in setting up, using, or maintaining the equipment, changes in performance, or to report unexpected operation or events. -
Page 22: Consumables And Accessories
2820 Eco BVF with Plastic Bite Lip (75) 9. Disposal The Vitalograph 2120 In2itive e-Diary is a clinical trial device and as such is returned to the manufacturer at the end of the study. Devices are appropriately disposed of in a separate collection. -
Page 23: Description Of The Vitalograph 2120 In2Itive E-Diary
15° from its normal position 11. Description of the Vitalograph 2120 In2itive e-Diary The device is a handheld spirometer with a diary function designed for use by trained professionals in a variety of environments for recording data, capture of patient reported outcomes, measuring and archiving tests on human subjects. -
Page 24: Indications For Use
Spirotrac application. There are a variety of configuration options available. Information about the device can be obtained from the About box. This information can be used when contacting Vitalograph or a service agent. To access the About box: Swipe down with two fingers on PIN entry screen. ... -
Page 25: Technical Specification
In2itive e-Diary - Instructions for Use 09180 Issue 2 12. Technical Specification Product Vitalograph 2120 In2itive e-Diary Fleisch type pneumotachograph capable of giving linear signals throughout the entire physiological range. During testing the airflow through the flowhead produces a pressure differential. - Page 26 A Global Leader in Respiratory Solutions Performance ATS/ERS 2019, ISO 23747:2015 & standards the device meets or ISO 26782:2009 exceeds EN 60601-1:2006+A1:2013 with US Safety standards deviations EMC Standards EN 60601-1-2:2015 Home use EN 60601-1-11:2015 standards QA/GMP EN ISO 13485, FDA 21 CFR 820, CMDR standards SOR/98-282 &...
- Page 27 In2itive e-Diary - Instructions for Use 09180 Issue 2 IP22 Ingress Protection 22 - the first digit indicates the level of protection that the enclosure provides against access to hazardous parts and solid foreign objects, the second digit indicates the level of...
-
Page 28: Contraindications, Warnings, Precautions And Adverse Reactions
1. No modification of this equipment is allowed. Any unauthorised changes to the device may compromise product safety and/or data and as such Vitalograph cannot be held responsible and the device will no longer be supported. 2. The device has no serviceable parts. The device must be returned to the manufacturer at the end of the Study. - Page 29 In2itive e-Diary - Instructions for Use 09180 Issue 2 between tests. The maximum number of registered tests allowed on device per session is 5, therefore patient fatigue is not likely. 13. Maintenance must not be performed while the device is in use by a patient.
- Page 30 26. Use of accessories and cables other than those specified or provided by Vitalograph for this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of the device and result in improper operation.
-
Page 31: Ce Notice
14. CE Notice Marking by the symbol indicates compliance of the Vitalograph 2120 In2itive e-Diary to the Medical Devices Directive of the European Community. The device is intended for use in a variety of professional healthcare and home environments, e.g. private domiciles, primary care,... - Page 32 A Global Leader in Respiratory Solutions EN 60601-1-2:2015 - Emissions tests Electromagnetic Emissions test Compliance environment - guidance The device uses RF energy only for its internal function. Therefore, its RF RF emissions Group 1 emissions are very low CISPR 11 and are not likely to cause any interference in nearby electronic equipment.
- Page 33 In2itive e-Diary - Instructions for Use 09180 Issue 2 ± 0.5 kV, ±1kV differential mode Device is identified Surge +/- 0.5 kV, 1kV as Class II unearthed IEC 61000-4-5 equipment therefore common mode testing is omitted. 100% drop, 0.5 cycles, 100% drop, 0.5 cycles,...
-
Page 34: Fda Notice
A Global Leader in Respiratory Solutions Use of accessories and cables other than those specified or provided by Vitalograph for this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of the device and result in improper operation. -
Page 35: Eu Declaration Of Conformity
09180 Issue 2 16. EU Declaration of Conformity Product: Vitalograph 2120 In2itive e-Diary Vitalograph hereby ensures and declares that the above product associated with these instructions for use, is designed and manufactured in accordance with the following QMS regulations and standards: European Medical Devices Directive {MDD} 93/42/EEC, as amended. -
Page 36: Guarantee
A Global Leader in Respiratory Solutions 17. Guarantee Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its option replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials. - Page 37 09180 Issue 2 not accept liability for any consequential damages arising out of the use of, or inability to use any Vitalograph® equipment. 8. This Guarantee is offered as an additional benefit to the Consumer’s statutory rights and does not affect these rights in any way.
- Page 38 A Global Leader in Respiratory Solutions Page of 40 DT_0006 Issue 15...
- Page 39 In2itive e-Diary - Instructions for Use 09180 Issue 2 Page of 40...















Need help?
Do you have a question about the In2itive e-Diary and is the answer not in the manual?
Questions and answers