Vitalograph In2itive e-Diary Instructions For Use Manual
Hide thumbs
Also See for In2itive e-Diary:
- Instructions for use manual (40 pages) ,
- Instructions for use manual (56 pages)
Summary of Contents for Vitalograph In2itive e-Diary
- Page 1 In2itive e-Diary MODEL 2120 Instructions for Use © Copyright Vitalograph 2020 Current Edition (Issue 1, 24-Feb-2020) Cat. No. 09140...
- Page 2 Technical Support Technical Support Tel: +353 65 6864111 Telefon: +49 40 547391-14 Email: technical.support@vitalograph.ie E-mail: support@vitalograph.de © Copyright Vitalograph 2020 Current Edition (Issue 1, 24-Feb-2020) Cat. No. 09140 Vitalograph is a registered trademark. Page of 28 DT_0006 Issue 15...
-
Page 3: Table Of Contents
Customer Service ..............12 Consumables and Accessories .........12 Disposal ................12 10. Explanation of Symbols ............13 11. Description of the Vitalograph In2itive e-Diary....14 11.1. Use in Clinical Trials..........14 11.2. Indications for Use ..........15 12. Technical Specification ............15 13. Contraindications, Warnings, Precautions and Adverse Reactions ..............17... -
Page 4: Main Components
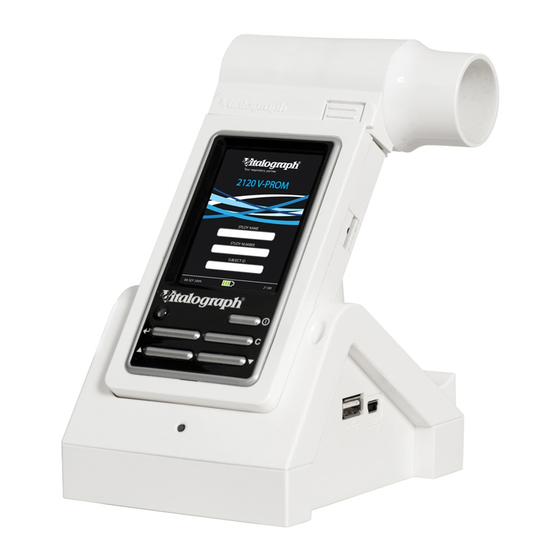
A Global Leader in Respiratory Solutions 1. Main Components Figure 1: Main Components of the Vitalograph In2itive e-Diary Note: Computer not supplied COMPACT Expert or PC running Spirotrac software Flowhead Flowhead Release Button LCD/Touch Panel Display Mini USB Ports Power Button (On/Off) -
Page 5: Features
Fleisch type pneumotachograph flowhead to measure flow. 2. Set Up The Vitalograph In2itive e-Diary is a clinical trial device and is set up by a healthcare professional for a particular subject prior to use. Any configuration required is downloaded to the device prior to issuing the device to the subject;... -
Page 6: Device Set Up
For full instructions and guidance on using the Vitalograph In2itive e-Diary with Spirotrac please refer to the customised study manual. 3. Operating Instructions Prior to use, the Vitalograph In2itive e-Diary should be set up and configured for subject use following instructions in the study manual. -
Page 7: Subject Use
The Vitalograph In2itive e-Diary is intended to be a single patient use device. If, for any reason, a device is used on more than one subject (E.g. -
Page 8: Power Management
Note: A USB port capable of supplying 500mA is required for charging over USB. The In2itive e-Diary emits an audible alarm when it is time to complete a session and the device turns on. If the subject misses the alarm, the device will automatically power down after a period of time, but the device may be turned on again by pressing the power button. -
Page 9: Automatic Power Down
Plug in the 5V Power Supply or USB cable to PC or Vitalograph Compact USB port to re-charge the batteries and continue testing.. 5. Cleaning & Hygiene 5.1. Hygiene and Cleaning The Vitalograph In2itive e-Diary is a single patient use device. At the Page of 28... -
Page 10: Inspection Of The In2Itive
70% isopropyl alcohol (IPA) impregnated cloth to remove any visible soiling and for low level disinfection. The screen may be cleaned with a soft cloth or cotton pad if required. The Vitalograph In2itive e-Diary is not designated as a sterile device. -
Page 11: Fault Finding Guide
In2itive e-Diary - Instructions for Use 09140 Issue 1 6. Fault Finding Guide Problem Fault • Test begins automatically Symptoms: • Volume accumulates automatically without the subject blowing. Possible Solutions: • Flowhead not stationary at start of test. (In probable order) Hold device steady until the ‘Blow Icon’... -
Page 12: Software Check
Test Data Storage Card 79166 Stylus (2) 9. Disposal The Vitalograph In2itive e-Diary is a clinical trial device and must be returned to the manufacturer at the end of the study. Used BVFs constitute minimally soiled waste from human Page... -
Page 13: Explanation Of Symbols
In2itive e-Diary - Instructions for Use 09140 Issue 1 healthcare and should be disposed of in line with local requirements. BVFs are made from polypropylene. 10. Explanation of Symbols Symbol Description Type BF equipment Class II Power rating Direct current Instructions for Use;... -
Page 14: Description Of The Vitalograph In2Itive E-Diary
Serial Number Power Button (On/Off) 11. Description of the Vitalograph In2itive e-Diary The Vitalograph In2itive e-Diary is a handheld spirometer which measures subject respiratory parameters including but not limited to VC, FVC, FEV1, PEF and MVV. The integral Fleisch flowhead is used for testing. -
Page 15: Indications For Use
In2itive e-Diary - Instructions for Use 09140 Issue 1 11.2. Indications for Use The indications for use of the Vitalograph In2itive e-Diary is in the assessment of lung function through the measurement of dynamic lung volumes, i.e. spirometry. The Vitalograph In2itive e-Diary is designed to be operated by medical professionals trained in respiratory and lung function testing on adults and paediatrics, 2.5 years and older, in a variety... - Page 16 10–40ºC Operating humidity 30%–75% range Ambient pressure range 850hPa–1060hPa Performance standards ATS/ERS 2005, ISO 23747:2009 & the Vitalograph In2itive ISO 26782:2009 e-Diary meets or exceeds Safety standards EN 60601-1: 2006 EMC Standards EN 60601-1-2: 2007 QA/GMP standards EN ISO 13485, FDA 21 CFR 820,...
-
Page 17: Contraindications, Warnings, Precautions And Adverse Reactions
Vitalograph cannot be held responsible and the device will no longer be supported. 2. The Vitalograph In2itive e-Diary has no serviceable parts. The Page of 28... - Page 18 5V power supply that is supplied with the device or via a PC/Compact USB port using the supplied USB cable. 5. The Vitalograph In2itive e-Diary is not designed as a sterile device. 6. Always follow the safety guidelines given by the manufacturer of cleaning and disinfectant chemicals.
- Page 19 500mA must be used or it will not charge the device fully or charging will take longer than 5 hours. 16. The Vitalograph In2itive e-Diary flowhead is intended to be a single patient use device. If, for any reason, a device is used on more than one subject (e.g.
-
Page 20: Ce Notice
24. Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Vitalograph In2itive e-Diary including cables specified by Vitalograph. Otherwise, degradation of the performance of this equipment could result. - Page 21 The customer or the user of the Vitalograph In2itive e-Diary should assure that it is not used in such an environment. The Vitalograph In2itive e-Diary has been tested in accordance with: EN 60601-1:2006- Medical electrical equipment.
- Page 22 3 V/m Radiated RF 3 V/m 80MHz to 2500 EN 61000-4-3 80MHz to 2500MHz Use of accessories and cables other than those specified or provided by Vitalograph for this equipment could result in increased Page of 28 DT_0006 Issue 15...
-
Page 23: Fda Notice
Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Vitalograph In2itive e-Diary including cables specified by Vitalograph. Otherwise, degradation of the performance of this equipment could result. -
Page 24: Eu Declaration Of Conformity
A Global Leader in Respiratory Solutions 16. EU Declaration of Conformity Product: Vitalograph In2itive e-Diary, Model 2120 Vitalograph hereby ensures and declares that the above product associated with these instructions for use, is designed and manufactured in accordance with the following QMS regulations... -
Page 25: Guarantee
In2itive e-Diary - Instructions for Use 09140 Issue 1 17. Guarantee Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its option replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials. - Page 26 A Global Leader in Respiratory Solutions not accept liability for any consequential damages arising out of the use of, or inability to use any Vitalograph® equipment. 8. This Guarantee is offered as an additional benefit to the Consumer’s statutory rights and does not affect these rights in any way.
- Page 27 In2itive e-Diary - Instructions for Use 09140 Issue 1 Page of 28...















Need help?
Do you have a question about the In2itive e-Diary and is the answer not in the manual?
Questions and answers