Summary of Contents for Vitalograph Pneumotrac 6800
- Page 1 Pneumotrac with Respiratory Muscle Strength (RMS) MODEL 6800 Instructions for Use © Copyright Vitalograph 2023 Current Edition (Issue 5, 24 Jan 2023) Cat. No. 09600...
- Page 2 Tel: +49 40 547391-40 Fax: +49 40 547391-40 E-mail: info@vitalograph.de www.vitalograph.com Technical Support Telefon: +49 40 547391-14 E-mail: technical.support@vitalograph.de © Copyright Vitalograph 2023 Current Edition (Issue 5, 24-Jan-2023) Cat. No. 09600 is a registered trademark. Page of 24 DT_0006 Issue 17...
-
Page 3: Table Of Contents
Contents Indications for Use ..............4 Contraindications, Warnings, Precautions and Adverse Reactions ..............4 Main Components of the Vitalograph Pneumotrac RMS ...7 3.1. Features of the Vitalograph Pneumotrac RMS ...8 Setting Up the Vitalograph Pneumotrac RMS ....8 Operating Instructions ............9 Power Management .............9 Cleaning &... -
Page 4: Indications For Use
1. No modification of this equipment is allowed. Any unauthorised changes to the Pneumotrac device may compromise product safety and/or data and as such Vitalograph cannot be held responsible and the device will no longer be supported. 2. The Pneumotrac is not designed as a sterile device. Always follow the safety guidelines given by the manufacturer of cleaning and disinfectant chemicals. - Page 5 Aneurisms c. Uncontrolled hypertension d. Urinary incontinence 16. If subject cannot form a good seal on the BVF during MIP/MEP test then a bite-on mouthpiece should be fitted to the BVF. 17. Service and repairs should be carried out only by the manufacturer or by Service Agents specifically approved by Vitalograph. Page of 24...
- Page 6 A Global Leader in Respiratory Diagnostics 18. Maintenance must not be performed while the device is in use by a subject. 19. Use of accessories and cables other than those specified or provided by Vitalograph for this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of the Pneumotrac and result in improper operation.
-
Page 7: Main Components Of The Vitalograph Pneumotrac Rms
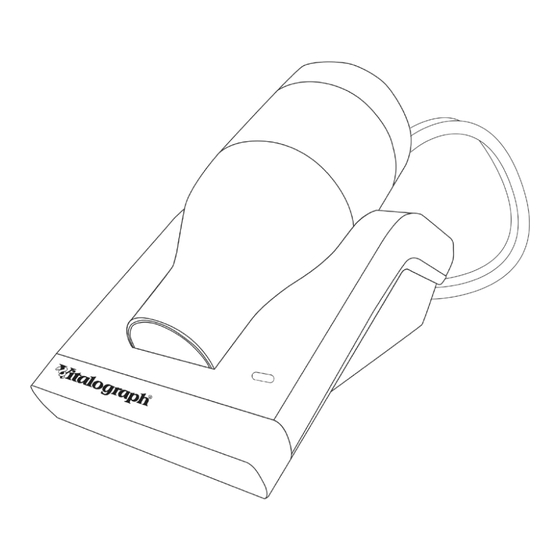
Pneumotrac with Respiratory Muscle Strength (RMS)- Instructions for Use 09600 Issue 5 3. Main Components of the Vitalograph Pneumotrac RMS Figure 1 USB Flash Drive containing Spirotrac USB Cable Pneumotrac Base Fleisch Flowhead Connection Tubing Fleisch Flowhead Bacterial Viral Filter (BVF) MIP or MEP Flowhead. -
Page 8: Features Of The Vitalograph Pneumotrac Rms
3. Browse USB Drive and click Setup. 4. Select Install Spirotrac. Follow on-screen instructions to complete installation. Further details are provided in the Spirotrac Instructions supplied with the software. 5. Close installation and select the Vitalograph Spirotrac icon from the desktop. Page of 24... -
Page 9: Operating Instructions
5. Operating Instructions The Pneumotrac RMS works with Vitalograph Spirotrac software. Spirotrac must be installed on the PC to begin testing. Refer to Spirotrac Instructions for Use for details on: •... -
Page 10: Cleaning & Hygiene
7. Cleaning & Hygiene 7.1. Preventing Cross-Contamination of Subjects A spirometer is not designed or supplied as a ‘sterile’ device. Vitalograph intends that a new Bacterial Viral Filter (BVF)/ SNIP nasal probe be used for every subject to prevent cross contamination. Using a new BVF provides a significant level of protection of the subject, the device and the user against cross contamination during spirometry manoeuvres. - Page 11 Pneumotrac with Respiratory Muscle Strength (RMS)- Instructions for Use 09600 Issue 5 Figure 3: Flowhead Assembly Flowhead Complete Flowhead End Cap Flow Conditioning Mesh Fleisch element Assembly ‘O’ Rings Pressure Tapping Flowhead Cone Lubrication: Silicone Grease MIP/MEP Flowhead: Visual inspection is recommended on a routine basis. Unscrew the MIP/MEP end cap from the cone.
- Page 12 A Global Leader in Respiratory Diagnostics MIP/MEP end cap MIP/MEP one way valve MIP/MEP cone One-way valve hard plastic housing Soft silicone one-way valve Figure 4: MIP/MEP Flowhead Assembly Carefully re-assemble the silicone one-way valve with the hard plastic housing, taking care not to damage the silicone one-way valve.
-
Page 13: Fault Finding Guide
Pneumotrac with Respiratory Muscle Strength (RMS)- Instructions for Use 09600 Issue 5 To check the MIP/MEP flowhead has been correctly re-assembled attach a BVF to the MIP/MEP Flowhead and test as follows: 1. MEP Flowhead: fully inspire though the flowhead. The one- way valve should open, and no resistance should be felt. Then attempt to expire. The one-way valve should close, and a significant resistance should be felt. The only air escaping should be through the MIP/MEP Pressure Vent on the flowhead. - Page 14 A Global Leader in Respiratory Diagnostics Possible • Flowhead and/or tubing not stationary at the start Cause/ of test. Hold them steady until the ‘Blow Now’ Solution: (In prompt appears. probable order) • Restart the test routine. Problem Fault • Rocking Pneumotrac Base Symptoms: Possible •...
-
Page 15: Customer Service
Service and repairs should be carried out only by the manufacturer, or by Service Agents approved by Vitalograph. Contact information for approved Vitalograph Service Agents may be found at the start of this manual. Any serious incident that has occurred in relation to the device should be reported to Vitalograph or its Authorized Representative and the Regulatory Authorities of the country. -
Page 16: Disposal
A Global Leader in Respiratory Diagnostics SNIP Nasal Probe (Pack of 30 – Small x 10, 32956 Medium x 10, Large x 10) 32981 SNIP Nasal Probe (Pack of 30 – Small) 32982 SNIP Nasal Probe (Pack of 30 – Medium) 32983 SNIP Nasal Probe (Pack of 30 –... -
Page 17: Device Description
QR code - matrix bar code. All information in the bar code is included in the text under it 13. Device Description The Vitalograph Pneumotrac RMS is a spirometry device which measures subject respiratory parameters as part of lung function testing. It is designed for desktop use. A Fleisch flowhead is used for spirometry testing and has a resting location on the device. -
Page 18: Ce Notice
Vitalograph Model 6800 Pneumotrac to the Medical Devices Directive of the European Community. The Vitalograph Model 6800 Pneumotrac is intended for use in a variety of professional healthcare environments, e.g. primary care, hospital wards and occupational health centres, except for... - Page 19 Pneumotrac with Respiratory Muscle Strength (RMS)- Instructions for Use 09600 Issue 5 requirements for basic safety and essential performance. EN 60601-1-2:2015 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests. EN 60601-1-2:2015- Emissions tests Emissions Electromagnetic environment -...
-
Page 20: Fda Notice
Medical Devices may be affected by mobile RF communications equipment including cellular telephones and other personal or household devices not intended for medical facilities. It is recommended that all equipment used near the Vitalograph product comply with the medical electromagnetic compatibility standard and to check before use that no interference is evident or possible. -
Page 21: Eu Declaration Of Conformity
09600 Issue 5 17. EU Declaration of Conformity Product: 6800 Vitalograph Pneumotrac Vitalograph hereby ensures and declares that the above product associated with these instructions for use, is designed and manufactured in accordance with the following QMS regulations and standards: •... -
Page 22: Guarantee & Free Five Year Warranty
A Global Leader in Respiratory Diagnostics 18. Guarantee & Free Five Year Warranty Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its option replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials. - Page 23 8. To the maximum extent permitted by law, the Company does not accept liability for any consequential damages arising out of the use of, or inability to use any Vitalograph equipment. ® 9. This Guarantee is offered as an additional benefit to the Consumer’s statutory rights and does not affect these rights in any way.















Need help?
Do you have a question about the Pneumotrac 6800 and is the answer not in the manual?
Questions and answers