Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Welch Allyn RETeval-DR
- Page 1 ® ™ Welch Allyn RETeval-DR Directions for use...
- Page 2 © 2018 Welch Allyn. All rights are reserved. To support the intended use of the product described in this publication, the purchaser of the product is permitted to copy this publication, for internal distribution only, from the media provided by Welch Allyn. No other use, reproduction, or distribution of this publication, or any part of it, is permitted without written permission from Welch Allyn.
-
Page 3: Table Of Contents
Connect the sensor strip lead ................15 Thumb joystick ....................15 Device settings ....................16 Operating instructions ................23 Turn the RETeval-DR on and off ................ 23 Perform a test ..................25 Testing overview ....................25 Prepare the device ..................... 25 Prepare the patient .................... - Page 4 Download the firmware update ................. 35 Copy the firmware to the device ............... 35 Update the firmware on the device ..............35 RETeval-DR utilities .................... 36 Clean and disinfect ................37 Clean and disinfect ..................... 37 Clean the ganzfeld ..................... 37 Troubleshooting ..................
-
Page 5: Introduction
For information about any Welch Allyn product, contact Welch Allyn at www.welchallyn.com/about/company/locations. Intended use The RETeval-DR device is intended to generate photic signals and measure and display evoked responses generated by the retina and the visual nervous system. The operators of the device are intended to be physicians, optometrists, medical technicians, clinical medical assistants, nurses, and other health-care professionals. -
Page 6: Latex Statement
Latex statement The components of the RETeval-DR device that could contact the user or patient were not made with natural rubber latex. This includes all items that could be contacted during normal operation, and all other functions, such as user maintenance and cleaning, as are defined in the Directions for use. -
Page 7: Symbols And Definitions
Consult operating instructions/directions for use (DFU). A copy of the DFU is available on this website. A printed copy of the DFU can be ordered from Welch Allyn for delivery within 7 calendar days. - Page 8 ® ™ 4 Symbols and definitions Welch Allyn RETeval-DR Miscellaneous symbols Manufacturer Date of manufacture Product Identifier Reorder Number Do not re-use By prescription or order of physician Type BF applied parts Meets essential requirements of European Medical Device Directive 93/42/EEC...
-
Page 9: Equipment Serial Number
Equipment serial number Each RETeval-DR device has a unique equipment serial number. The equipment serial number may appear in one of two formats. The serial number takes the form R # # # # # #. Product code is R... - Page 10 ® ™ 6 Equipment serial number Welch Allyn RETeval-DR...
-
Page 11: About Warnings And Cautions
Warning and caution statements can appear on the Welch Allyn RETeval-DR device, the packaging, the shipping container, or in this Directions for use. The RETeval-DR is safe for patients and clinicians when used in accordance with the instructions and the warning and caution statements presented in this Directions for use. - Page 12 CAUTION This device is not protected against the ingress of water and should not be used in the presence of liquids which may enter the device. CAUTION Do not connect the RETeval-DR device to the docking station while measuring a patient. This will compromise the quality of recordings and subject isolation.
- Page 13 Directions for use About warnings and cautions 9 CAUTION Only use the cleaning or germicidal cleaner agent types listed or damage may occur.
- Page 14 ® ™ 10 About warnings and cautions Welch Allyn RETeval-DR...
-
Page 15: Controls And Connectors
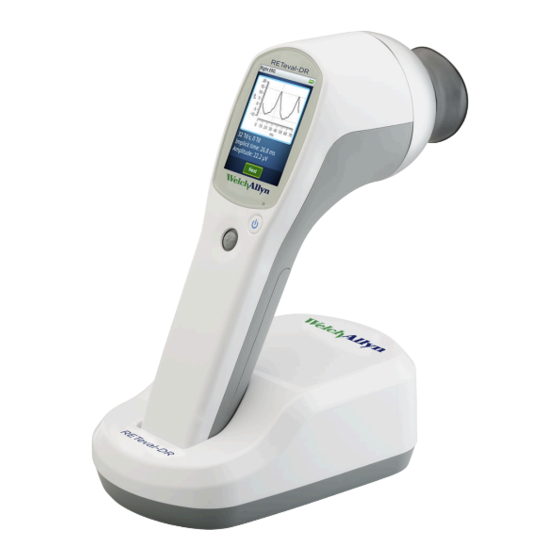
RETeval-DR device Used to test for diabetic retinopathy Docking station Charges RETeval-DR and enables data transfer to a PC. Connect to an electrical outlet using the supplied power brick LCD display Displays information for charging, testing, and adjustments to the... - Page 16 Allows you to move through menus, perform functions, and select parameters by moving the joystick up/down/right/left/select Eyecup Blocks ambient light during testing Bezel Area to which the eyecup attaches Sensor strip lead connector Connects the RETeval-DR device to the sensor strip Battery cover Covers the battery compartment...
-
Page 17: Setup
Setup Unpack the system The RETeval-DR device is packaged with the following items. Confirm that all items are included. Number Item Description RETeval-DR device Used to test for diabetic retinopathy. Sensor strip lead Connects the device to sensor strips for testing... -
Page 18: Docking Station
Charge the battery When the RETeval-DR device battery charge is low, a warning message is shown on the device screen. Return the device to the docking station and let it charge. Do not attempt to test a patient after this message appears. -
Page 19: Connect The Sensor Strip Lead
USB connection is used. The charging status is shown on the display. If the display is blank, press the power button to turn it on. The RETeval-DR device is shipped with a partial charge. -
Page 20: Device Settings
® ™ 16 Setup Welch Allyn RETeval-DR Press the joystick... To... Move the selection up DOWN Move the selection down LEFT when the cursor is at the left edge of the screen Go back one screen RIGHT when the cursor is at the right edge of the screen... - Page 21 Scanning is automatic and does not require the joystick to be pressed. Follow the instructions in the "RETeval-DR Utilities" section to download and install software utilities for the device.
- Page 22 RETeval-DR device. The RETeval-DR device can store up to 50 test results. You must remove old results to make room for new results. There are three ways to remove results.
- Page 23 (Maa et al. 2016). The default reference interval for RETeval-DR is set at 7.0-19.9; this reference interval can be adjusted to trade off sensitivity, specificity, and negative predictive values by varying the upper point of the reference interval, as shown in the examples below.
- Page 24 ® ™ 20 Setup Welch Allyn RETeval-DR The default lower point of the reference interval is set at 7.0 and is three standard deviations below the mean for the patients without retinopathy (Maa et al. 2016); typically, the lower point of the reference interval is not changed.
- Page 25 Directions for use Setup 21 Return device to initial factory condition Returning your device to its initial factory condition erases everything from the device, including patient information, test results, and settings. To do a “factory reset”, you need to do both “Erase everything” and “Reset settings”. CAUTION Results deleted on the device cannot be recovered.
- Page 26 ® ™ 22 Setup Welch Allyn RETeval-DR...
-
Page 27: Operating Instructions
Operating instructions Turn the RETeval-DR on and off CAUTION Do not turn off the device while saving data from a patient test. Turning off the device while saving patient data may delete the data. 1. Press the Power button to turn the device on. - Page 28 ® ™ 24 Operating instructions Welch Allyn RETeval-DR...
-
Page 29: Perform A Test
Prepare the device 1. Remove the RETeval-DR device from the docking station; the device turns on automatically. If the device is not in the docking station, press the Power button to power on the device. -
Page 30: Prepare The Patient
WARNING Patient injury risk. Dispose of single-use components (for example, sensor strips) after using them one time. RETeval-DR sensor strips are to be used for a single examination only. Sensor strips may not adhere well if re-used, causing an excessively high electrode impedance and potentially preventing the device from producing a result. -
Page 31: Device Testing Options
Skips the eye displayed for testing Test each eye The RETeval-DR device is designed to measure the patient’s right eye first. The default is to test both eyes. If you only want to measure a patient’s left eye, use the Skip button to proceed past the right eye screen without testing the patient. -
Page 32: Remove The Sensor Strips
® ™ 28 Perform a test Welch Allyn RETeval-DR At the beginning of each test, the device automatically Note recalibrates the light intensity and color, during which time the patient will see brief red, green, and blue flashes. This process takes about one second. If recalibration is unsuccessful, an “Unable to calibrate”... -
Page 33: Test Results
Test results The DR Assessment Protocol combines implicit time, amplitude, age, and pupil response. The protocol creates a unified result score, which is shown immediately after test completion. Patients with more-severe levels of diabetic retinopathy will typically have a higher test result (Maa et al. 2016). The DR Assessment Protocol will classify the test result as either High, Normal, or Low depending upon the reference interval selected. -
Page 34: View Results
30 Test results Welch Allyn RETeval-DR The image above shows the dependence of RETeval-DR measurements on diabetic retinopathy severity level. Plots show the mean and standard error of the mean for each severity group listed in the table below. Severity group definitions... - Page 35 Directions for use Test results 31 Two periods of the electrical response, as measured from the Sensor strip, to a 32 Td∙s (left) and 16 Td∙s (right) white flickering stimulus are shown. The light flashes stimulating the retina occurred at time = 0 ms and near time = 35 ms. The dotted lines indicate the measurement points for the peak-to-peak amplitude and implicit time (time-to-peak).
-
Page 36: Delete Results From The Device
RETeval-DR device. The RETeval-DR device can store up to 50 test results. You must remove old results to make room for new results. There are three ways to remove results. - Page 37 1. Press the Power button to power on the RETeval-DR device. 2. Scroll down to Settings > Memory > Erase all test results, and then press the joystick.
- Page 38 ® ™ 34 Test results Welch Allyn RETeval-DR...
-
Page 39: Manage Firmware And Software
Update firmware Periodically Welch Allyn publishes an update to the device firmware. You must download the update to the computer or laptop first, connect the RETeval-DR device to the computer or laptop, and complete the firmware update process. Download the firmware update Find firmware updates at www. -
Page 40: Reteval-Dr Utilities
36 Manage firmware and software Welch Allyn RETeval-DR If the RETeval-DR update fails, verify that the firmware update file was downloaded and copied to the device correctly by repeating “Copy the firmware” and “Update the firmware on the device.” RETeval-DR utilities... -
Page 41: Clean And Disinfect
Welch Allyn recommends that you clean the eyecup and sensor strip lead between each patient. The RETeval-DR device is chemically compatible to wipes containing 70% isopropyl alcohol and with wipes containing alkyl dimethyl benzyl ammonium chloride. - Page 42 ® ™ 38 Clean and disinfect Welch Allyn RETeval-DR...
-
Page 43: Troubleshooting
Start test button, I get an “The pupil can no longer be found” error The RETeval-DR device measures the pupil size and adjusts the brightness of the flickering light for each flash based upon the pupil size. The Start test button is only... - Page 44 (making the timing faster). The eyecup is designed to block external light from reaching the eye. If the RETeval-DR device senses too much ambient light, an error message will display on the screen. After pressing Restart to reduce the amount of ambient light reaching the eye, perform the following.
- Page 45 A small change in the light reflectance of the ganzfeld will create a large change in the color or intensity of the light output, which is corrected by this recalibration. If the correction is too large, the RETeval-DR device will create an error.
- Page 46 ® ™ 42 Troubleshooting Welch Allyn RETeval-DR...
-
Page 47: Specifications
Specifications Specifications Light source Red LED (621 nm) Green LED (530 nm) Blue LED (470 nm) White (RGB) Flash 0.0001 – 15 0.001 – 17 0.0001 – 5 0.002 – 30 luminance energies (cd∙s/m Background 0.03 – 3000 0.2 – 3500 0.03 –... - Page 48 Welch Allyn will service RETeval-DR devices that are within their lifetime. Support may require an annual subscription service after the initial one (1) year warranty period. The expected battery life is at least one (1) year. If the RETeval-DR device fails to hold a charge, a new battery can be ordered.
-
Page 49: Maintenance
Maintenance User maintenance The RETeval-DR device contains no user serviceable parts other than the eyecup and battery, both of which can be replaced without the need of tools. To maintain proper function and compliance to regulatory requirements, do not attempt to disassemble the device. -
Page 50: Calibration And Storage
RETeval-DR Calibration and storage Item Description Calibration The RETeval-DR device includes automated internal flash calibration and QC checks. No testing can be carried out by users. Storage Store the device in the docking station and place the dust cover over the device when not in use. -
Page 51: Standards And Compliance
Standards and compliance General compliance and standards Many local laws and regulations require special procedures to recycle or dispose of electrical equipment-related waste including batteries and other elements of electronic devices. Follow all of your respective local laws and regulations for the proper disposal of batteries and any other parts of this system. - Page 52 Welch Allyn RETeval-DR Guidance and Manufacturer’s Declaration – Emissions The RETeval-DR is intended for use in the electromagnetic environment specified below. The customer or user of the RETeval-DR should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment–...
- Page 53 70 % UT; 25/30 cycles for 70 % UT; 25/30 cycles for interruptions, it is recommended 50 Hz and 60Hz, 50 Hz and 60Hz, that the RETeval-DR be powered respectively respectively from an uninterruptible power Single phase: at 0° Single phase: at 0°...
- Page 54 Recommended Separations Distances for the RETeval-DR device The RETeval-DR device is intended for use in the electromagnetic environment in which radiated disturbances are controlled. The customer or user of the RETeval-DR device can help prevent electromagnetic interference by maintaining a minimum distance between...
-
Page 55: Appendices
Pairs per package), Charging Dock, and USB Cable; 100-240 V, 50-60 Hz, Lithium-Ion Battery (#RETeval-ACC-02); IEC Plug Types A, G, E/F, and I (RETeval-ACC-05); English Directions for RETeval-SS-50 Disposable Sensor Strips for Welch Allyn RETeval-DR Visual Electrodiagnostic Device; Quantity 50 Pairs RETeval-ACC-04 Eyecup Accessory for Welch Allyn RETeval-DR Visual Electrodiagnostic Device RETeval-ACC-01 Lead-Wire Accessory for Welch Allyn RETevall-DR Visual Electrodiagnostic Device RETeval-ACC-05 Power Transformer Accessory Kit for Welch Allyn RETeval-DR Visual Electrodiagnostic Device;... - Page 56 ® ™ 52 Appendices Welch Allyn RETeval-DR least one eye. The average testing time for the RETeval-DR device during the clinical trial was 2.3 minutes to test both eyes. Severity group definitions International clinical classification ETDRS level CSME (Wilkinson et al. 2003)
- Page 57 If the patient is on medications or has other conditions that impair the pupil response, extra care must be taken to properly interpret the RETeval-DR device results, as these individuals are more likely to be erroneously classified as likely to have vision threatening DR. Further, ensure the contralateral eye is covered by the patient’s hand to prevent uncontrolled light...
-
Page 58: Warranty
This warranty does not cover damage caused by: 1) attempted disassembly or repair by anyone not authorized by Welch Allyn, 2) failure to follow instructions for use and maintenance, or 3) accidental drops or impacts. If the RETeval-DR product covered... - Page 59 Welch Allyn will, at its discretion, repair or replace the product free of charge. You must obtain a return authorization from Welch Allyn to arrange for the return of your RETeval-DR product before you send it to the designated Welch Allyn service center for repair or replacement.












Need help?
Do you have a question about the RETeval-DR and is the answer not in the manual?
Questions and answers