Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Hill-Rom MetaNeb System
- Page 1 The MetaNeb® System User Manual Product No. PMN4 174432 REV 7...
-
Page 3: Revision
Rom makes no commitment to update or keep current, the information in this manual. Hill-Rom reserves the right to make changes without notice in design, specifications, and models. The only warranty Hill-Rom makes is the express written warranty extended on the sale or rental of its products. - Page 4 MetaNeb® and MetaTherapy® are registered trademarks of Comedica, Inc. Replace this manual (174432) if it is damaged and/or can not be read. For product support or to order additional copies of this manual (174432), contact your distributor, local Hill-Rom representative, or go to www.hill- rom.com. Reference Documents MetaNeb®...
-
Page 5: Table Of Contents
Table of Contents Table of Contents Revision ............i Document Symbols . - Page 6 Table of Contents MetaTherapy® Treatment Protocol ....... 16 Frequency ..........16 Procedure .
-
Page 7: Document Symbols
Document Symbols DOCUMENT SYMBOLS This manual contains different typefaces and symbols to make the content easier to read and understand: • Standard text—used for regular data. • Boldface text—emphasizes a word or phrase. • NOTE:—sets apart special data or important instruction clarification. •... -
Page 8: Indications
Indications INDICATIONS Indications for Use The MetaNeb® System is indicated for mobilization of secretions, lung expansion therapy, the treatment and prevention of pulmonary atelectasis, and also has the ability to provide supplemental oxygen when used with compressed oxygen. Patient Population •... -
Page 9: Precautions
Indications Precautions • Federal law restricts this device to sale by or on the order of a physician. • Circuits are for single patient use, multiple treatment sessions. • Do not occlude entrainment orifices. • Do not use on uncooperative patients. •... -
Page 10: Introduction
Introduction INTRODUCTION The MetaNeb® System supplies a therapy that enhances secretion removal and helps prevent or resolve patchy atelectasis. Description The MetaNeb® System is a therapeutic device that uses a systematic approach to enhance normal mucus clearance and resolve or prevent patchy atelectasis. -
Page 11: Theory Of Operation
Introduction Theory of Operation Normal Mucus Clearance Normal mucus clearance in the lungs is accomplished in three unique ways. It must be understood that these mechanisms complement each other and none are mutually exclusive. The three mechanisms are: 1. Mucociliary Escalator 2. -
Page 12: Autocephalad Flow
Introduction Autocephalad Flow When gas flows over a thickly lined mucus layer, a shear force directly proportional to the velocity of the gas is produced. If this airflow velocity is maintained and exceeds the cohesive forces of the mucus, the mucus will move in the direction of the gas flow. -
Page 13: The Metaneb® System User Manual (174432 Rev
Introduction CPEP is a therapy which supplies a continuous, clinician-set airway pressure above atmospheric by use of a venturi, a fixed orifice resistor, and flow during both inspiration and expiration. CPEP— • Prevents or reverses atelectasis. • Delivers hyperinflation therapy through positive expiratory pressure that will help patients deeply breathe and cough. -
Page 14: Features
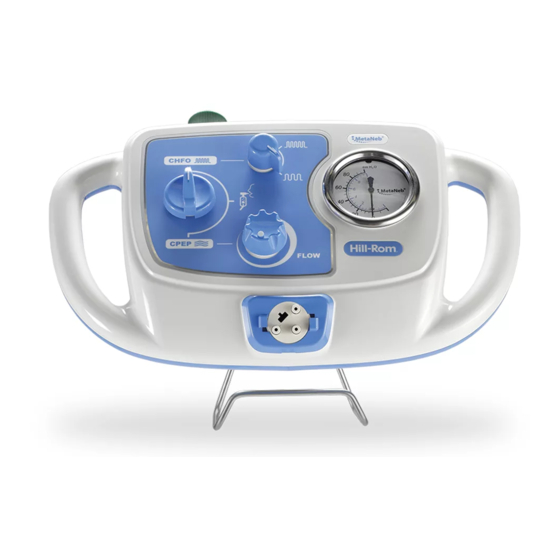
Features FEATURES Controller Item Description Item Description Pressure manometer CPEP flow adjuster Higher/lower switch Master on/off switch Mode selector Oxygen gas connector Circuit tri-connector Mount bracket port The MetaNeb® System User Manual (174432 REV 7) -
Page 15: Circuit
Features Circuit Item Description Item Description Mouthpiece Orifice indicators Selector ring Adapter, 22 mm x 15 mm Handset Adapter, 22 mm x 22 mm Circuit tri-connector/ Occlusion ring bio-filter Tubing Silicon adapter Nebulizer Nebulizer tubing The MetaNeb® System User Manual (174432 REV 7) -
Page 16: Assemble The Controller System
Assemble the Controller System ASSEMBLE THE CONTROLLER SYSTEM Make sure you have all the parts that are listed on the contents list. Stand Assembly and Controller Installation NOTE: Some older devices may have a different stand. For a stand that differs from that shown, refer to the instructions that were supplied with the stand. -
Page 17: Assemble The Circuit
Assemble the Circuit ASSEMBLE THE CIRCUIT Typical Mouthpiece Configuration Put the circuit tri-connector/bio-filter into the tri-connector port on the control unit. Turn the connector 45° counterclockwise to lock it into position. Remove the mouthpiece from the package. Attach the mouthpiece to the handset: insert the mouthpiece at a 45° angle and gently push it in and twist it to the correct orientation. -
Page 18: Other Configurations
Assemble the Circuit Other Configurations Mask Tracheostomy Flex tube adapter not included In-Line with Ventilator Circuit “T”connector not included The MetaNeb® System User Manual (174432 REV 7) -
Page 19: Add Medication To The Nebulizer
Assemble the Circuit Add Medication to the Nebulizer CAUTION: The maximum capacity of the nebulizer container is 5 mL. Do not fill the container beyond the maximum fill indication point (5 mL). To do so could cause equipment damage. 5 mL 1 mL Remove the container cap (1) from the nebulizer. -
Page 20: Pre-Use Check
30 cm H 11. If the device is not within the parameters specified above, do not use the unit. Contact Hill-Rom Technical Support to examine and repair the unit. The MetaNeb® System User Manual (174432 REV 7) -
Page 21: Pre-Use Check For In-Line Use With A Ventilator
20 and not more than 45 cm H 10. If the device is not within the parameters specified above, do not use the unit. Contact Hill-Rom Technical Support to examine and repair the unit. -
Page 22: Metatherapy® Treatment Protocol
MetaTherapy® Treatment Protocol METATHERAPY® TREATMENT PROTOCOL Frequency The common strategy for frequency of MetaTherapy® Treatment, in the acute care setting, ranges from two (2) to four (4) times daily. The patient’s response to the therapy should determine any frequency adjustments. Procedure Make sure the unit operates correctly. -
Page 23: Assessment Of Outcome
MetaTherapy® Treatment Protocol 16. Encourage the patient to exhale slowly (3-4 seconds). 17. Continue CPEP mode approximately 2 ½ minutes, adjusting the flow to achieve a therapy that is comfortable yet challenging for the patient or as otherwise provided in institutional protocol. 18. -
Page 24: The Metaneb® System In-Line With Ventilator Protocol
The MetaNeb® System In-Line with Ventilator Protocol THE METANEB® SYSTEM IN-LINE WITH VENTILATOR PROTOCOL WARNING: Only persons trained to use the The MetaNeb® System and ventilators should perform therapy on ventilated patients. Frequency In-line use of The MetaNeb® System with a ventilator ranges in frequency from four (4) to eight (8) times daily as determined by the patient's response to the therapy. -
Page 25: Assessment Of Outcome
The MetaNeb® System In-Line with Ventilator Protocol 10. Set the mode to CHFO and select Higher. 11. Put the master switch in the ON position. Higher 12. Put a spring-valve "tee" adapter into the inspiratory limb of the ventilator circuit. 13. -
Page 26: Move The Stand
Move the Stand MOVE THE STAND Disconnect the oxygen hose from the facility connection, and put the hose around the hanger bracket. Unlock the two locking casters. Move the stand to the applicable location. Lock the two casters. Connect the oxygen hose to the facility connection. CLEANING WARNING: Failure to follow these cleaning instructions could cause injury or... -
Page 27: Cleaning The Controller And Stand
Cleaning WARNING: Do not use phenolic, alcohol, quaternary ammonium chloride, or bleach solutions to clean the patient circuit. Use these solutions to clean the controller only. Injury or equipment damage can occur. The Metaneb® Controller has been tested for compatibility with the following cleaning and disinfecting solutions: Chemical Class Active Ingredient... - Page 28 Cleaning WARNING: To help prevent cross-contamination, replace the SPU circuit between patients. Failure to do so could cause infection. WARNING: Do not use phenolic, alcohol, quaternary ammonium chloride, or bleach solutions to clean the patient circuit. Use these solutions to clean the controller only.
- Page 29 Cleaning NOTE: Before the next use of the system, do these: • Examine the nebulizer for cracks or other damage. If there is damage, replace the circuit. Refer to “Circuit Part Numbers” on page 38. • Assemble the nebulizer. Refer to “Add Medication to the Nebulizer”...
-
Page 30: Troubleshooting And Maintenance
Troubleshooting and Maintenance TROUBLESHOOTING AND MAINTENANCE Troubleshooting Problem Examine Repair Circuit will not Examine the O-rings. Replace the O-rings. connect correctly. Examine the Replace the unit if the connector port. port is damaged. Circuit will not Circuit not connected. Disconnect the circuit, function. -
Page 31: Maintenance
Units returned for maintenance and repair must be handled by Hill-Rom, and must have a return goods authorization (RGA) number. For disposal of the controller, return it to Hill-Rom or your Hill-Rom distributor. Shipping and Packaging When The MetaNeb® System controller is shipped for repair, follow these... -
Page 32: Product Symbols
Product symbols PRODUCT SYMBOLS Symbol Definition Consult accompanying documents. Continuous Positive Expiratory Pressure (CPEP) mode supplies medicated aerosol combined with continuous positive pressure to help hold open and expand the airways. Continuous High Frequency Oscillation (CHFO) mode is a pneumatic form of chest physiotherapy that delivers medicated aerosol while oscillating the airways with continuous pulses of positive pressure. - Page 33 Product symbols Symbol Definition This product is not manufactured with natural rubber latex. Manufacturer and date of manufacture Non-sterile product By Prescription Only (USA only) Single patient use product The MetaNeb® System conforms to the European Medical Device Directive 93/42/EEC Authorized European Representative a.
-
Page 34: Specifications
Specifications SPECIFICATIONS Feature Dimension Controller (also see page 29) Weight 4.8 lb (2.2 kg) Height x width x depth 10" x 15" x 4" (254 x 381 x 102 mm) Bracket Arm Weight 1.2 lb (.5 kg) Height When attached to the standard 1" (2.5 cm) diameter pole, the height is adjustable throughout the pole length Stand (also see page 30) -
Page 35: Controller Dimensions
Specifications Controller Dimensions The MetaNeb® System User Manual (174432 REV 7) -
Page 36: Stand Dimensions
Specifications Stand Dimensions Environmental Conditions for Transport and Storage Condition Range Temperature -40° to 158°F (-40° to 70°C) ambient temperature Relative humidity 10% to 95% non-condensing Environmental Conditions for Use Condition Range Temperature 50° to 94°F (10° to 35°C) ambient temperature Relative humidity range 10% to 95% non-condensing... -
Page 37: Classification And Standards
Technical Specification for Performance—Breathing Simulator LASSIFICATION AND TANDARDS Technical and Quality Assurance ISO 13485 EN60601-1 EN13544-1 FDA Medical Device Equipment Class II Classification Classification According to Medical Device Directive 93/42/EEC TECHNICAL SPECIFICATION FOR PERFORMANCE—BREATHING SIMULATOR The performance checks shown on page 32 were measured by use of a breathing simulator. -
Page 38: Chfo Pulses-Airway Pressure Reading
Technical Specification for Performance—Breathing Simulator CHFO Pulses—Airway Pressure Reading CHFO Results CHFO High Rate 240 ± 60 bpm (300 bpm max) CHFO Low Rate 70% of CHFO High Rate (100 bpm min) Peak Pressure 30 cmH2O Oscillating Amplitude 5 cmH2O minimum CPEP—Airway Pressure Reading The MetaNeb®... -
Page 39: Hi Pressure Chfo Pulses-Airway Pressure Reading
Technical Specification for Performance—Breathing Simulator Hi Pressure CHFO Pulses—Airway Pressure Reading CHFO Results CHFO High Rate 240 ± 60 bpm (300 bpm max) CHFO Low Rate 70% of CHFO High Rate (100 bpm min) Peak Pressure 40 cmH2O Oscillating Amplitude 5 cmH2O minimum Hi Pressure CPEP—Airway Pressure Reading The MetaNeb®... -
Page 40: Bench Performance Checks-Breathing Simulator
Bench Performance Checks—Breathing Simulator BENCH PERFORMANCE CHECKS—BREATHING SIMULATOR The performance checks shown on page 35 through page 37 were measured by use of a breathing simulator. WARNING: Due to excessive restriction of valves in the Controller, reduced performance levels may result when gas supply pressures <... - Page 41 Bench Performance Checks—Breathing Simulator Adult Norm Breathing Simulator Settings—Bench Check 1 Lung Resistance 5 cmH2O/l/s Lung Compliance 70 mL/cmH2O Breath Rate 15 bpm Effort Amplitude 15cmH2O Effort Slope Effort % Inhale Effort Offset 0 cmH20 Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples...
- Page 42 Bench Performance Checks—Breathing Simulator Adult Obstructive Breathing Simulator Settings—Bench Check 2 Lung Resistance 5 cmH2O/l/s Lung Compliance 90 mL/cmH2O Breath Rate 15 bpm Effort Amplitude 15cmH2O Effort Slope Effort % Inhale Effort Offset 0 cmH20 Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples...
- Page 43 Bench Performance Checks—Breathing Simulator Adult Restrictive Breathing Simulator Settings—Bench Check 3 Lung Resistance 30 cmH2O/l/s Lung Compliance 25 mL/cmH2O Breath Rate 15 bpm Effort Amplitude 15cmH2O Effort Slope Effort % Inhale Effort Offset 0 cmH20 Target Volume 650 mL Flow Conversion Factor Time between Samples 30.1 milliseconds Number of Samples...
-
Page 44: Nebulizer Performance
NOTE: The performance of the nebulizer may differ from that shown below. The performance is dependent on the type of drug and nebulizer unit that is used. For more information, contact Hill-Rom, your distributor, or the drug supplier. NOTE: The performance data shown below may not be applicable for suspensions or medications with a high viscosity. -
Page 45: Particle Specifications
Particle Specifications PARTICLE SPECIFICATIONS The following specifications were established through performance tests using an eight-stage cascade impactor at a flow rate of 28 liters per minute (LPM) equipped with a USP <601> induction port throat. Three device samples were tested with three runs each for a total of nine sample points per each drug for a total of 27 data points. - Page 46 Particle Specifications CHFO Mode at 28 LPM Particle Specifications with 95% Confidence Level for 1 Cycle Particle Drug MetaNeb Characterization MMAD (um) Albuterol 0.83-1.19 Ipratropium 0.51-1.00 Cromolyn 0.59-0.85 2.79-3.75 2.76-4.01 2.55-3.335 Total Dose Delivered by 421.6-572.4 Device ug 124.5-204.6 1367.9-1903.9 Total Respirable Dose 139.2-200.2 (0.5 - 5 um)
- Page 47 Particle Specifications CPEP Mode at 28 LPM Particle Specifications with 95% Confidence Level for 1 Cycle Particle Drug MetaNeb Characterization MMAD (um) Albuterol 0.71-1.12 Ipratropium 0.33-0.83 Cromolyn 0.27-0.44 3.99-4.76 3.23-6.93 3.77-5.04 Total Dose Delivered by 389.6-529 Device ug 149-180.1 647.5-929.9 Total Respirable Dose 89-137.8 (0.5 - 5 um)
- Page 48 Particle Specifications NOTES: The MetaNeb® System User Manual (174432 REV 7)













Need help?
Do you have a question about the MetaNeb System and is the answer not in the manual?
Questions and answers