Subscribe to Our Youtube Channel
Summary of Contents for Roscoe Medical SoundCare Plus
- Page 1 INSTRUCTION MANUAL SoundCare™ Plus Pain Relief Device Operating instructions Medical device for pain treatment based on ultrasound www.roscoemedical.com...
-
Page 2: Table Of Contents
SoundCare™ Plus Pain Relief Device Contents 1. FOREWORD…………………………………………………..…...3 2. SAFETY PRECAUTIONS…………………………………………...3 3. INDICATIONS AND CONTRAINDICATIONS…………………...6 4. PRESENTATION…………………………………………………..7 5. INSTALLATION…………………………………………………… 10 6. OPERATION……………………………………………………...13 7. MAINTENANCE……………..…………………………………...15 8. DIAGNOSTICS………………………………….………………..16 9. SPECIFICATIONS AND TECHNICAL DATA…………..……….. 17 10.STORAGE ………………………………………………………...18 11.DISPOSAL……………………...……………………..……..18 12.STANDARDS AND REQUIREMENTS………….………………...19 13.GUIDANCE AND MANUFACTURER'S DECLARATION …..….. 19 14.WARRANTY…………………………………………………….…23 15.NORMALIZED SYMBOLS……………………………..…………24... -
Page 3: Foreword
This user manual is published by Roscoe Medical, Inc. Roscoe Medical, Inc. does not guarantee its contents and reserves the right to improve and amend it at any time without prior notice. Amendments will however be published in a new edition of this manual. - Page 4 SoundCare™ Plus Pain Relief Device Warning: Text with a “WARNING” indicator will explain possible safety infractions that will potentially cause serious injury and equipment damage. Danger: Text with a “DANGER” indicator will explain possible safety infractions that are imminently hazardous situations that would result in death or serious injury.
- Page 5 SoundCare™ Plus Pain Relief Device Warning WARNING To avoid the risk of electric shock, the SoundCare™ Plus must only be connected to a supply mains with protective earth. Care must be taken when operating this equipment around other equipment. Potential electromagnetic or other interference could occur to this device or to the other equipment.
-
Page 6: Indications And Contraindications
SoundCare™ Plus Pain Relief Device Injury, damage, or death can occur during diathermy therapy even if the implanted neurostimulation system is turned “off.” Indications and Contraindications 1) Application of therapeutic deep heat can be used for the treatment Indications of selected sub-chronic and chronic medical conditions such as: •... -
Page 7: Presentation
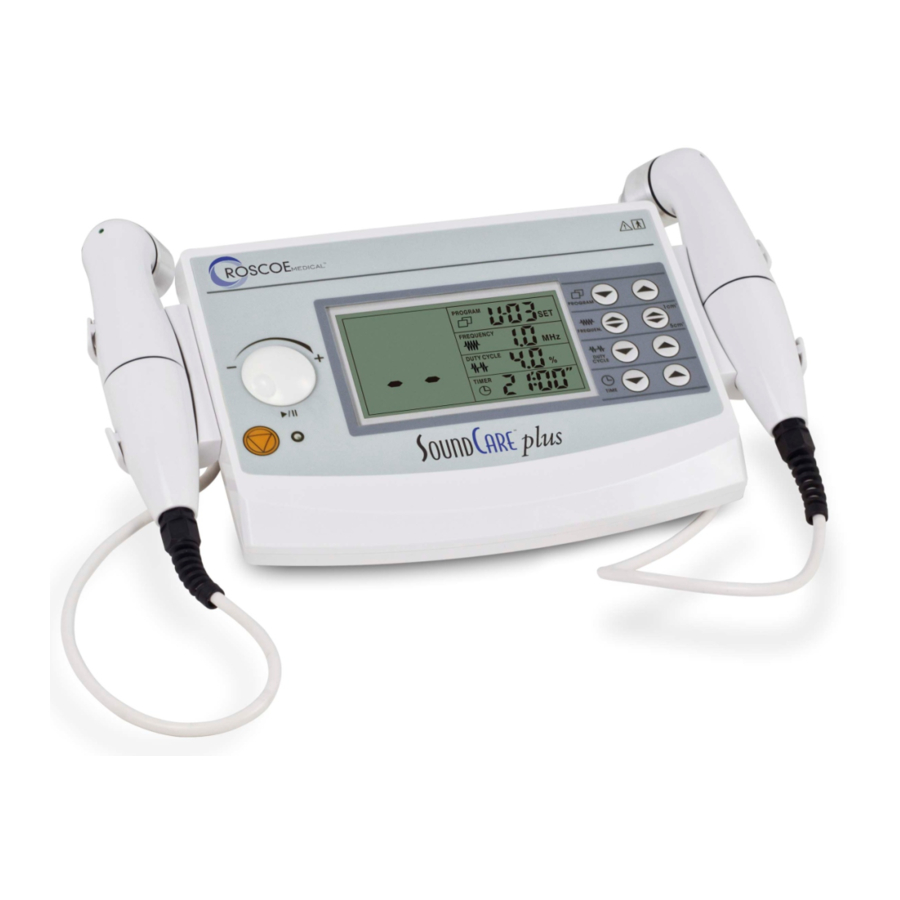
SoundCare™ Plus Pain Relief Device PRESENTATION Presentation of the device PROGRAM FREQUENCY POWER DUTY CYCLE TIMER 1) Program adjustment button 2) 1MHz and 3MHz adjustment button 3) Duty cycle adjustment button 4) Timer adjustment button 5) Liquid crystal display 6) Stop button 7) Output intensity adjustment knob and pause button 8) Treatment head select button 9) The socket of treatment head (5cm ) - Page 8 SoundCare™ Plus Pain Relief Device Liquid crystal PROGRAM display FREQUENCY POWER DUTY CYCLE TIMER Output intensity/power 1) Program indicator Frequency indicator Ultrasound output indicator Pause indicator Duty cycle indicator Treatment head type Timer indicator Treatment head 1) Sound head The component of the Applicator which makes contact with the patient during the Ultrasound therapy treatment of the time.
- Page 9 SoundCare™ Plus Pain Relief Device Below are the definitions for all of the symbols used in the Ultrasound Symbol therapy device . Study and learn these symbols before any operation definitions of the system. ON/OFF SWITCH STOP TREATMENT START / PAUSE BUTTON ULTRASOUND INTENSITY ULTRASOUND APPLICATOR STATE POLARITY OF POWER SUPPLY...
-
Page 10: Installation
SoundCare™ Plus Pain Relief Device UP and DOWN Arrows Controls used in various modality parameter screens to navigate or Ø change a value up or down within the parameter. INSTALLATION Remove the Therapy System and all accessories from shipping cartons. Reception Visually inspect for damage. - Page 11 SoundCare™ Plus Pain Relief Device PROGRAM PROGRAM FREQUENCY FREQUENCY POWER Figure 2 Figure 1 DUTY CYCLE DUTY CYCLE TIMER TIMER Caution:SoundCare™ Plus can only use the applicator that attached with main unit by the manufacturer. Please do not use other type of applicator. Switch on the device, using the power switch.
- Page 12 SoundCare™ Plus Pain Relief Device 5.5.3 Select the treatment head 1cm or 5cm by pressing the treatment Choice/ head select button. Select the treatment head 1cm or 5cm CAUTION: The user can select the treatment head 1cm or 5cm by pressing the treatment head select button when the two types of treatment head have been connected with the device.
-
Page 13: Operation
SoundCare™ Plus Pain Relief Device Switch off the SoundCare™ Plus, by pressing the main switch. Pull out the main plug of the mains adapter from the adapter Receptacle. Disconn- ecting from the mains OPERATION Measures with regard to treatments 6.1.1 Put the patient in a comfortable position. - Page 14 SoundCare™ Plus Pain Relief Device Warning If you want to replace the treatment head, you need to turn the power switch off, so that the device is in shutdown state in order to replace the treatment head. 6.1.3 Clean the treatment area well before treatment as well as the treatment After the -head by using a towel or a tissue.
-
Page 15: Maintenance
SoundCare™ Plus Pain Relief Device In order to ensure efficient transfer of energy, a contact medium is The contact required between the treatment head and the body. Air causes virtually medium total reflection of the ultrasound energy. The best medium for the transfer of ultrasound energy is a gel. -
Page 16: Diagnostics
SoundCare™ Plus Pain Relief Device The treatment heads and cables should be regularly inspected for damage, Cleaning e.g. hairline cracks, which could allow penetration by liquids. Clean the of the contact surface immediately after each treatment. Make sure that no treatment ultrasound gel remains on the treatment head. -
Page 17: Specifications And Technical Data
SoundCare™ Plus Pain Relief Device Check the connected plug of ultrasound applicator. The contact PROGRAM control fails to operate FREQUENCY Figure 4 DUTY CYCLE TIMER SPECIFICATIONS AND TECHNICAL DATA Technical data of device Electrical Acoustic Frequency: 1MHz±10%,3MHz±10% data Output Power: ulse repetition rate 100Hz±10% Duty factor :... -
Page 18: Storage
SoundCare™ Plus Pain Relief Device Environmental conditions for use ℃ Environment temperature: 10-40 Relative humidity: 30%-85% Atmospheric pressure: 800-1060hPa Environmental conditions for storage ℃ ℃ Environment temperature: -10 -55 Relative humidity: 10%-90% Atmospheric pressure: 700-1060hPa Duty Suggest Program Frequency Treatment Program Cycle Time... -
Page 19: Standards And Requirements
SoundCare™ Plus Pain Relief Device Roscoe Medical, Inc. declares that the device complies with STANDARDS following normative documents: IEC/EN60601-1, IEC/EN60601-1-2, IEC/EN60601-2-5, REQUIREMENTS IEC/EN61689, IEC60601-1-4,IEC62304,ISO10993-5, ISO10993-10, ISO10993-1,ISO14971. GUIDANCE MANUFACTURER'S DECLARATION 13.1 Guidance and manufacturer's declaration - electromagnetic Declaration- emissions electro- The SoundCare™ Plus device is intended for use in the electromagnetic magnetic environment specified below. - Page 20 SoundCare™ Plus Pain Relief Device 13.2 Guidance and manufacturer's declaration-electromagnetic Declaration - immunity electromagnetic The SoundCare™ Plus device is intended for use in the electromagnetic immunity environment specified below. The customer or the user of the SoundCare™ Plus should assure that it is used in such an environment. Immunity Electromagnetic Compliance...
- Page 21 SoundCare™ Plus Pain Relief Device where P is the maximum output power rating of the transmitter in watts (W) according to the Transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey, should be less...
- Page 22 SoundCare™ Plus Pain Relief Device 13.4 RF communications equipment and the SoundCare™ Plus device Recommended Recommended separation distances between portable and mobile separation distances RF communications equipment and the SoundCare™ Plus device between The SoundCare™ Plus device is intended for use in an electromagnetic environment portable in which radiated RF disturbances are controlled.
-
Page 23: Warranty
SoundCare™ Plus Pain Relief Device Please contact your dealer or the device centre in case of a claim under WARRANTY the warranty. If you have to send in the device, enclose a copy of your receipt and state what the defect is. A. -
Page 24: Normalized Symbols
SoundCare™ Plus Pain Relief Device NORMALIZED SYMBOLS The name and the address of the manufacturer Type BF Applied Part Serial Number of product Batch code Please attention to adjust the effective intensity Disposal in accordance with Directive 2002/96/EC (WEEE) Only for treatment head: Protected against the effects of IPX7 temporary immersion water Complies with the European Medical Device Directive... - Page 28 Manufacturer for Roscoe Medical, Inc. 21973 Commerce Parkway, Strongsville, Ohio 44149 www.roscoemedical.com 0197...











Need help?
Do you have a question about the SoundCare Plus and is the answer not in the manual?
Questions and answers