Table of Contents
Advertisement
OMRON ULTRASONIC
NEBULISER
NE-U17
Model
Instruction Manual
■ Thank you very much for purchasing this OMRON
Ultrasonic Nebuliser.
■ Be sure to read this instruction manual carefully
before using the unit so that you can use it safely and
correctly.
■ Please keep this instruction manual always at hand
for future reference.
(NE-U17-E)
■ This unit is a medical instrument. Be sure to use the unit properly
Table of Contents
Be sure to Read This Section
About the Product
How to Use the Unit
How to Take Care of the Unit
Specifications
according to instructions given by medical professionals.
Only use medications as prescribed and instructed by your doctor.
Exemptions from Liabilities ......... 1
Intended Use .............................. 2
Notes on Safety .......................... 3
Features of the Product .............. 6
Composition of the Product ........ 7
Names of the Parts .................... 8
before Inhalation ...................... 9
How to Inhale ........................... 11
(Using Optional Parts) ........... 13
How to Take Care of the Unit ... 15
How to Disinfect the Unit .......... 17
How to Store the Unit ............... 18
Troubleshooting ........................ 19
Specifications ........................... 20
List of Optional Parts ................ 22
IM-U17-E-03-09/2014
1615886-5G
EN
DE
NL
FR
IT
ES
Advertisement
Table of Contents

Summary of Contents for Omron NE-U17
-
Page 1: Table Of Contents
(NE-U17-E) Table of Contents Instruction Manual Be sure to Read This Section ■ Thank you very much for purchasing this OMRON Exemptions from Liabilities ..1 Ultrasonic Nebuliser. Intended Use ......2 ■ Be sure to read this instruction manual carefully Notes on Safety ...... -
Page 2: Exemptions From Liabilities
However, if you find any omission or error, please inform your local OMRON representative or dealer. 3. It is prohibited to copy all or a part of this Instruction Manual without getting OMRON’s permission. Unless you use this Instruction Manual for your personal (corporate) purpose, you are not allowed to use it without OMRON’s permission in accordance with the Copyright... -
Page 3: Intended Use
Intended Use Medical Purpose This product is intended to be used for inhaling medication for respiratory disorders or for humidification in combination with oxygen therapy. Intended User healthcare personnel or patient under the guidance of qualified medical experts. operation and the content of instruction manual. Intended Patients This product should not be used by patients, who are unconscious or are not breathing spontaneously. -
Page 4: Notes On Safety
Be sure to immediately dry the cleaned and disinfected parts, then store them in a dry place in order to prevent them from getting contaminated in the future. In case of any problem with your nebuliser please contact your local OMRON service representative (address on/ inside package). - Page 5 Do not hold the inhalation hose during nebulisation. Do not disassemble, repair, or modify the unit. Contact your nearest OMRON service representative. Do not use a power cord other than the supplied one. Be sure to use the power source as specified on the device.
- Page 6 (1) Power-off the unit and unplug the power plug from the electric outlet to make the unit inoperable. (2) Write “Faulty-Do not use” on the main unit so that it will not be used. (3) Contact the store where you purchased the unit or the nearest OMRON dealer.
-
Page 7: Features Of The Product
About the principle of Aerosol= Nebulised medication nebulisation in the NE-U17 ➀ The NE-U17 transmits the energy of ultrasonic vibration from the vibrator located at the bottom of the water tank into water. ➁ The ultrasonic vibration is transmitted to the medication in the medication cup through the water in the water tank. -
Page 8: Composition Of The Product
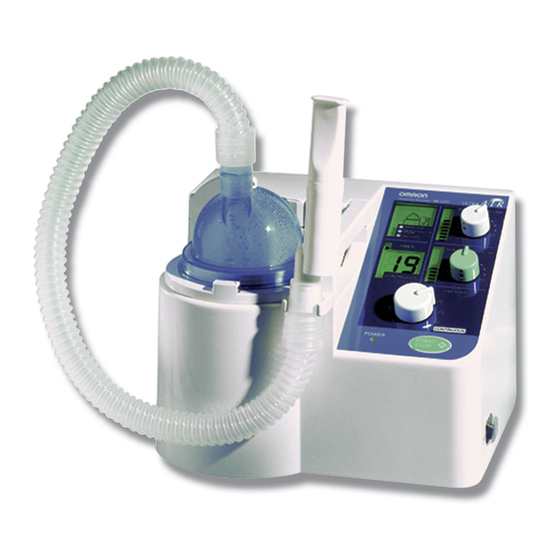
Composition of the Product Inhalation hose (M) with a cuff, 70 cm Mouthpiece set Two medication cups Main unit ■ Instruction manual (with warranty card) Power cord – As the unit is a Class I electroshock protection type, be sure to use only the supplied power cord with an “earth”. -
Page 9: Names Of The Parts
Names of the Parts * indicates replaceable supplies. Medication cup cover Air duct cover Washer Fan cover Mouthpiece set Air filter case Air filter* Handle Inhalation hose M Display of air volume Medication cup* (Refer to the “Description of the LCD Display” on Page 7.) Display Air volume adjustment knob Display of nebulisation rate... -
Page 10: How To Prepare The Unit Before Inhalation
How to Prepare the Unit before Inhalation – Clean the parts before starting the inhalation. Filling the cooling water. [Parts that can be disinfected by boiling] (For the disinfecting method, refer to Page 17.) Submerge these parts in a container filled with 1) Remove the nebulisation section sufficient water and boil for 10 minutes. - Page 11 How to Prepare the Unit before Inhalation Filling medication into the medication Assembling and attaching the cup. nebulisation unit to the main unit 1) Remove the medication cup cover 1) Fix the nebulisation unit by rotating by rotating it counterclockwise. the medication cup cover-fixing lever counterclockwise.
-
Page 12: How To Inhale
How to Inhale Setting the inhalation time. Push the START/STOP Button ( The fan will rotate and the unit starts Setting the inhalation time by rotating to nebulise. the timer knob clockwise. 1) To set the timer The timer can be adjusted by 1 minute when setting the time up to 15 minutes. - Page 13 How to Inhale Inhaling the aerosol. Finishing the treatment. <Example of correct use> When the timer is used: The buzzer sound notifies the completion of time set by the timer and the unit stops operation. When the operation ends, “0” on the display illuminates and the display changes to the initial set time.
-
Page 14: How To Inhale (Using Optional Parts)
How to Inhale (Using Optional Parts) Using the unit for a long time By using the optional “long time nebulisation set (4997217-7)” and the “supply medication set (4997206-1)”, the unit can nebulise continuously for a long time. ➀ Remove the medication cup cover of the main unit. Attach an optional long time nebulisation set in place of the medication cup cover and assemble the unit according to the procedure shown on Page 10. - Page 15 How to Inhale (Using Optional Parts) Oxygen supply pipe Using a bacterial filter Having passed through a bacterial filter, the filtered air can be supplied to the inside of the long time nebulisation set. ➀ Remove the medication cup cover and assemble the long time nebulisation set with the main unit. To assemble, use the long time nebulisation set in place of the medication cup cover by following the procedure shown on Page 10.
-
Page 16: How To Take Care Of The Unit
How to Take Care of the Unit How to Take Care of the Parts Turn off the power by pushing the ON/ OFF Button ( ), and unplug the power plug from the electric outlet. Remove the mouthpiece set, or inhalation mask. - Page 17 How to Take Care of the Unit How to Take Care of the Air Filter Replace the air filter when it becomes dirty. ➀ Remove the nebulisation unit from the main Nebulisation device. section ➁ Remove the fan cover by lifting it up. ➂...
-
Page 18: How To Disinfect The Unit
How to Disinfect the Unit How to Disinfect the Unit Disinfection by boiling cannot be done to some parts. They may become deformed. [Parts that can be disinfected by boiling] Medication Washer cup cover Inhalation hose (M) with a cuff, 70 cm [Parts that cannot be disinfected by boiling] Air filter case Air duct cover... -
Page 19: How To Store The Unit
How to Disinfect and Store the Unit Sterilization with ethylene oxide gas (E.O.G.) Observe the Instruction Manual of the sterilizer and consult with the manufacturer of the E.O.G. sterilizer for the details. Some of the rubber and plastic parts of the nebuliser may have residual ethylene oxide gas. After sterilization, consult with the manufacturer of the sterilizer and provide sufficient aeration until the safe level to human beings can be obtained. -
Page 20: Troubleshooting
E1 is displayed. Contact the nearest OMRON dealer. stopped. – If the unit does not operate normally after taking the above-mentioned measures, do not touch the internal mechanism and consult the store where you purchased the unit or the nearest OMRON dealer. -
Page 21: Specifications
Result of cascade impactor measurements for particle size individual mean Dp (μm) * Independently measured at SolAero Ltd., Canada, Dr. John Dennis, according to EN13544-1:2007+A1:2009. ** Measured by OMRON HEALTHCARE Co., Ltd. Notes: URL: www.omron-healthcare.com EN13544-1:2007+A1:2009, Respiratory therapy equipment - Part1: Nebulising systems and their components. Symbols:... - Page 22 EN60601-1-2:2007 standard has been implemented. This standard defines the levels of immunity to electromagnetic interferences as well as maximum levels of electromagnetic emissions for medical devices. This medical device manufactured by OMRON Healthcare conforms to this EN60601-1-2:2007 standard for both immunity and emissions.
-
Page 23: List Of Optional Parts
List of Optional Parts Optional Medical Accessories (within the scope of EC Medical Device Directive 93/42/EEC) Medication cup Bacterial filter Air filter set Air filter (4997211-8) (4997213-4) (4997214-2) (4997215-0) Used to transmit the ultrasonic Used for inhalation such as for Protects the unit from the dust in air. - Page 24 Manufacturer OMRON HEALTHCARE Co., Ltd. 617-0002 JAPAN EU-representative OMRON HEALTHCARE EUROPE B.V. Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS www.omron-healthcare.com OMRON HEALTHCARE Co., Ltd. Matsusaka Factory Production facility 1855-370, Kubo-cho, Matsusaka-shi, Mie, 515-8503 Japan OMRON HEALTHCARE UK LTD. Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK www.omron-healthcare.co.uk...
















Need help?
Do you have a question about the NE-U17 and is the answer not in the manual?
Questions and answers