Table of Contents
Advertisement
Quick Links
PROFESSIONAL MEDICAL PRODUC TS
KURA TENS - 6 PROGRAMMES
Use and maintenance book
AD-2126 (GIMA 28431)
ANDON HEALTH CO., LTD, No. 3 Jinping Street,
YaAn Road, Nankai District, Tianjin 300190, China
Made in China
iHealthLabs Europe SAS, 36
Rue de Ponthieu, 75008, Paris, France
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
105kPa
90%
%
80kPa
0%
60˚C
-20˚C
0197
Advertisement
Table of Contents

Summary of Contents for Gima AD-2126
- Page 1 PROFESSIONAL MEDICAL PRODUC TS KURA TENS - 6 PROGRAMMES Use and maintenance book AD-2126 (GIMA 28431) ANDON HEALTH CO., LTD, No. 3 Jinping Street, YaAn Road, Nankai District, Tianjin 300190, China Made in China iHealthLabs Europe SAS, 36 Rue de Ponthieu, 75008, Paris, France Gima S.p.A.
-
Page 2: Transcutaneous Electrical Nerve Stimulators
(TENS device) Model:AD-2126 Please read this user manual carefully before using the product Thank you for purchasing AD-2126 TENS device. Transcutaneous Electrical Nerve Stimulators (TENS device) is effective in relieving pain. Before using, please read the instructions carefully, so that you... -
Page 3: Table Of Contents
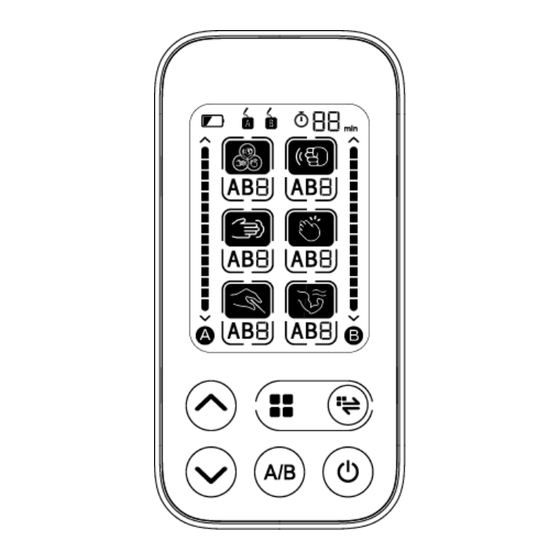
MODEL AD-2126 TENS device (Transcutaneous Electrical Nerve Stimulators) OPERATION GUIDE __________________________________________________________________________ INDEX INTENDED USE ....................3 CONTRAINDICATION ..................3 OPERATION PRINCIPLE ................3 CONTENTS AND DISPLAY INDICATORS ............. 3 PACKAGE CONTENTS .................. 4 SPECIFICATIONS ................... 4 NOTICE ......................5 SETUP AND OPERATING PROCEDURES ............ 6 1. -
Page 4: Intended Use
INTENDED USE The TENS device is intended to provide temporary relief of muscle soreness caused by exercise, normal household or work activities, as well as to alleviate chronic, intractable pain and pain associated with arthritis. It is important to apply the electrode pads only on intact skin and avoid placing them directly on the head, upper neck, chest, upper back near the heart, spine, and private areas. -
Page 5: Package Contents
4 Electrode Pads SPECIFICATIONS 1. Product name: Transcutaneous Electrical Nerve Stimulators (TENS device) 2. Model: AD-2126 3. Classification: Internally powered, Type BF applied part,IP22,No AP or APG, Continuous operation Machine size: Approx.120.3mm×60.3mm×20.6mm( 4 3/4″x 2 3/8″x 13/16″) Weight: Approx. 73g (2 9/16 oz.) (exclude batteries) Electrode Pads: Approx.50mm ×... -
Page 6: Notice
NOTICE Read all of the information in the operation guide and any other literature in the box before operating the unit. This TENS device is designed for adults and never should be used on infants or young children. Consult your physician or other health care professionals before use on older children. -
Page 7: Setup And Operating Procedures
Simultaneous connection of a PATIENT to a high frequency. surgical ME equipment may result in burns at the site of the stimulator electrodes and possible damage to the stimulator. Operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy EQUIPMENT may produce instability in the STIMULATOR output. -
Page 8: Prepare The Adhesive Gel Pads
The negative terminal of the battery needs to be compressed into the battery compartment properly after horizontal compression of the negative electrode. The battery is in contact with the spring. Make sure the battery cover is intact and not damaged before installing the battery. The monitor, the batteries the electrode pad, and the wire must be disposed of according to local regulations at the end of their usage. - Page 9 The Transcutaneous Electrical Nerve Stimulators (TENS device) can treat many different types of pain. On the next page are diagrams of where to place the electrodes for the most common forms of pain. For other areas of pain, place the electrodes on either side of the area of pain.
-
Page 13: Useing Your Tens Device
5. USEING YOUR TENS device Attach the adhesive gel pads around the area of pain. Press the ON/OFF ( ) button for one second to turn on the device. Note: you can press the “ON/OFF” button for one second in anytime to turn off the monitor manually when the unit is working . - Page 14 Composite(3) Output pulse frequency = 1~33.33Hz; Output pulse width =20~100μs; Composite(4) Output pulse frequency = 20~50Hz; Output pulse width =20~100μs; Thump(1) Output pulse frequency = 1~16.67Hz ; Output pulse width =20~100μs; Continue output Thump(2) Output pulse frequency = 1~16.67Hz; Output pulse width =20~100μs;...
- Page 15 Slap(1) Output pulse frequency = 5~20Hz; Output pulse width =20~100μs; Slap(2) Output pulse frequency = 2.5~10Hz; Output pulse width =20~100μs; Slap(3) Output pulse frequency = 3.33~5Hz; Output pulse width =20~100μs; Slap(4) Output pulse frequency = 3.33~10Hz; Output pulse width =20~100μs; Acupuncture(1) Output pulse frequency = 2.5~100Hz;...
-
Page 16: Troubleshooting
Muscular Relaxation(4) Output pulse frequency = 16.67~100Hz; Output pulse width =20~100μs; Press button to adjust output intensity of selected channel. When the output intensity is at 1~15 other 0, the treatment starts and the time setting counts down with a flashing time sign on the LCD display. The defaulted treatment time is 15 minutes. -
Page 17: Maintenance
off in the 2. Are the wire disconnected? 2. Turn off the power and connect the therapeutic 3. Have the batteries been wire. process. exhausted? 3. Please replace them with new ones. MAINTENANCE Do not drop this monitor or subject it to strong impact. Avoid high temperature and solarization. -
Page 18: Electromagnetic Compatibility Information
Serial number Manufacturer Date of manufacture Lot number Atmospheric pressure limit Temperature limit Humidity limit Medical Device compliant with Directive 93/42/EEC Authorized representative in the European community Follow instructions for use Product code Covering Protection rate WEEE disposal ELECTROMAGNETIC COMPATIBILITY INFORMATION Table 1 - Emission limits per environment Phenomenon Compliance... - Page 19 ±15kV air 10V/m Radiated RF EM field IEC 61000-4-3 80MHz-2.7GHz 80% AM at 1kHz Proximity fields from RF wireless IEC 61000-4-3 Refer to table 3 communications equipment Rated power 30A/m frequency magnetic IEC 61000-4-8 50Hz or 60Hz fields Table 3 - Proximity fields from RF wireless communications equipment Test frequency Band Immunity test levels...
- Page 20 Disposal: The product must not be disposed of along with other domestic waste. The users must dispose of this equipment by bringing it to a specific recycling point for electric and electronic equipment. GIMA WARRANTY TERMS The Gima 12-month standard B2B warranty applies.














Need help?
Do you have a question about the AD-2126 and is the answer not in the manual?
Questions and answers