Table of Contents
Advertisement
Quick Links
ACKeRmAnn FusIon
user
Integrated smart
Integrated smart
manual
16-2009
16-2009
16-2009
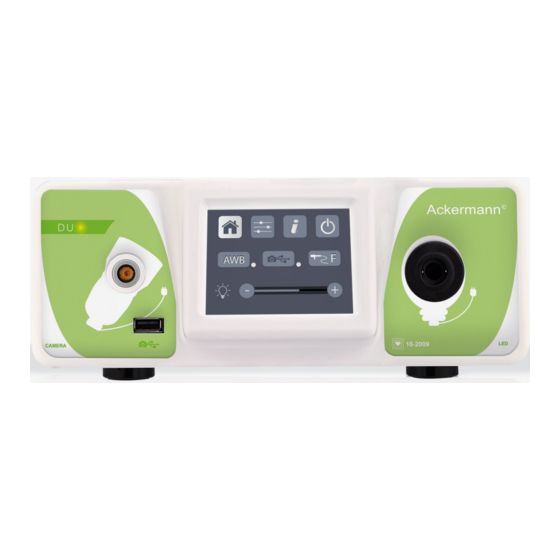
Duo Camera
» The DUO Full HD control unit is a medical device
combining a Full HD camera and LeD light source
over a single control channel. It is designed to provide
local illumination of the patient's body and to capture
images taken by health practitioners qualified
in diagnostic procedures or surgical endoscopy
procedures. «
Ackermann
technology
technology
User Manual 16 -2009 | Revision 01/20
Advertisement
Table of Contents

Summary of Contents for ACKERMANN 16-2009
- Page 1 Ackermann ACKeRmAnn FusIon user Integrated smart Integrated smart technology technology manual 16-2009 16-2009 16-2009 Duo Camera » The DUO Full HD control unit is a medical device combining a Full HD camera and LeD light source over a single control channel. It is designed to provide local illumination of the patient’s body and to capture...
-
Page 2: Table Of Contents
Due to the continuing progress and development of our products, we reserve all rights for technical alterations. © by: ackermann Instrumente gmbH Rev. 01/2020... -
Page 3: Foreword
You will find it on the product label and labels on the packaging. In this user manual, ackermann does not use any text, brand names, pictures, figurative signs or other items liable to mislead the user or patient over the purpose, safety and performance of the device. - Page 4 iNNOVATING TRADITION. as soon as you receive your device, you are asked to print and download all documents or sections of documents that you may need to consult in the event of an emergency, if you are unable to connect to the Internet or if your electronic display device (computer, tablet, etc.) stops working.
-
Page 5: Required Information
Universal light cable adaptors, source end (ackermann, Storz fitting) and endoscope end (ackermann, Storz fitting) (optional) Note: any other consumable or accessory not sold by ackermann will have its own manual. Please refer to it before using the product. Keep the packaging in case you need to transport the equipment at a later date. - Page 6 iNNOVATING TRADITION. Camera head The camera head included in the DUO Full HD unit is designed to relay the image captured at the examination site to a monitor via the control channel. It is designed for use by health practitioners qualified in diagnostics and surgical endoscopy procedures (gynaecology, laparoscopy, arthroscopy, eNT, urology or spinal endoscopy).
- Page 7 Ackermann User Manual 16 -2009 | Revision 01/20 by sPeCIFIC useR tRAInInG No specific training other than initial professional training is required to use this medical software. The practitioner is responsible for performing clinical treatments and for dangers that may arise due to a lack of skill and/or training.
- Page 8 iNNOVATING TRADITION. BAsIC PeRFoRmAnCe as stated in the applicable safety standard pertaining to electrical medical devices, the manufacturer has determined that the medical device does not manage basic performance. The active part, the camera head, is held by the practitioner throughout the entire medical procedure. as a highly skilled medical expert, the practitioner can immediately detect any problems at the treatment area and react accordingly.
-
Page 9: Safety Instructions
Look out for warning signs such as a lack of information, information in a foreign language, very attractive prices, suspect appearance, mediocre quality or premature wear. - Page 10 iNNOVATING TRADITION. PReCAutIons DuRInG use Unplug the device from the mains supply if you have no plans to use if for a few days or longer. To unplug the power cord, pull on the plug. Never pull on the cord itself. ...
- Page 11 Ackermann User Manual 16 -2009 | Revision 01/20 by PARtICulAR WARnInGs This device is not sterile; only the camera and lens may be sterilised. When connecting the light cable or camera to the control channel, do not force the connectors, as this may ...
-
Page 12: Installation Of The Equipment
innovAtInG tRADItIon. InstAllAtIon oF the Chapter equIPment ConneCtIon Connect the power cord to the cable connector at the rear of the device [C7] . Connect a video lead to one of the following video outputs: HDMI [C4] or SDI [C6]. ... - Page 13 CABle Connect the camera head connector [C8] to the control channel [C1]. Connect the light cable to the control channel [C2]. The device has two types of connections: ackermann (, Storz®) by default on the device. Olympus®, provided on the rear of the device [D1].
-
Page 14: Operating Principle
iNNOVATING TRADITION. Chapter oPeRAtInG PRInCIPle 5.1 stARtInG When the device starts up you will be taken to a standby screen. Press the touch screen [L1] to access the main screen for your device. mAIn sCReen WhIte BAlAnCe To adjust the white balance, proceed as follows: Once the camera and light source are connected to the endoscope, film a white surface (e.g. - Page 15 Ackermann User Manual 16 -2009 | Revision 01/20 by FoCussInG Use the focussing ring [F1] on the sensor lens to focus. Once the endoscope is connected and the source activated, slowly turn the ring until it is in a position ...
- Page 16 innovAtInG tRADItIon. moDe seleCtIon: RIGID oR FleXIBle The camera is in rigid mode by default. This mode must be used if you are using an endoscope equipped with lenses. If you are using a flexible endoscope equipped with optical fibre, change over to flexible mode: If you are using a flexible endoscope equipped with optical fibre, change over to flexible mode: ...
- Page 17 Ackermann User Manual 16 -2009 | Revision 01/20 by ADjustInG the settInGs On the header row, click the Settings button to go to the video settings screen These are the available settings: . Brightness . Sharpness . Red gain .
- Page 18 . Date/Time: takes you to a screen where you can adjust the date and time (2) . Settings: takes you to a screen where you can adjust system settings (3) ContACt SerViCe addreSS ackermann Instrumente gmbH eisenbahnstr. 65-67 78604 Rietheim-Weilheim germany...
- Page 19 Ackermann User Manual 16 -2009 | Revision 01/20 by DAte/tIme To adjust the date, touch the first rectangle at the top of the screen (marked DD/MM/YYYY). Then press the digits to complete the date. To adjust the time, touch the second rectangle at the ...
- Page 20 innovAtInG tRADItIon. Button [s3] AnD [s4] settInGs The Information>Settings menu includes a line with settings for the Zoom -/+ or LeD -/+ buttons. By default the setting selected is LeD -/+, but you can change it at any time to Zoom -/+ by pressing the ...
-
Page 21: Disinfection And Sterilization
CleAnInG AnD DIsInFeCtIon oF the CAmeRA heAD For the accessories listed in chapter 2, of which ackermann is not the manufacturer, please see the relevant manufacturer’s manual. For the accessories listed in chapter 2 and manufactured by ackermann, cleaning and disinfection guidance is given below. - Page 22 TRADITION. CAmeRA heAD steRIlIsAtIon For the accessories listed in chapter 2, of which ackermann is not the manufacturer, please see the relevant manufacturer’s manual. For the accessories listed in chapter 2 and manufactured by ackermann, sterilisation guidance is given below.
- Page 23 Devices may be sterilised in STeRIS® V-PRO® or STeRRaD® 100NX sterilisation systems using a Standard cycle. ackermann accepts use of the following sterilisation systems to reach the desired sterility assurance level (SaL) of 10-6: STeRIS® V-PRO® and STeRRaD® 100NX on a Standard cycle.
- Page 24 Use only polypropylene sterilisation packaging and/or polyolefine bags approved by the FDa. Do not use paper bags or sterilisation envelopes containing wood pulp or cotton. Comment: The devices judged by ackermann to be compatible with the STeRRaD® sterilisation process have been approved with a minimum of one hundred STeRRaD® cycles. steRRAD 100nX InstRuCtIons ®...
- Page 25 Do not use paper bags or sterilisation envelopes containing wood pulp or cotton. Comment: The devices judged by ackermann to be compatible with the STeRIS® sterilisation process have been approved with a minimum of one hundred STeRIS® cycles InstRuCtIons FoR the use oF steRIs v-PRo ®...
- Page 26 To prevent damage to devices, do not immerse them in a disinfectant solution for more than one hour. ackermann recommends the use of a cleaning agent and sterilisation method to prevent unknown rates of deterioration of materials due to interactions between the material and chemical products arising from different cleaning and sterilisation processes.
-
Page 27: After-Sales Service And Routine Maintenance
via a request on the website: www.ackermanninstrumente.de/contact.html ackermann will, at the request of technical personnel working for the network of approved dealers, provide all information required to repair the faulty parts on which they may perform repairs. Page:... - Page 28 iNNOVATING TRADITION. FAults AnD WARnInGs WaRNINg POSSIBLe CaUSe aCTION TO TaKe ON CONTROL UNIT ON MONITOR Light cable No light cable Light cable missing Connect a light cable disconnected Camera head Camera disconnected + No camera Connect the camera disconnected target on screen aWB indicator red aWB Not OK...
-
Page 29: Electromagnetic Compatibility
The recommended separation distances in this chapter must therefore be strictly observed. The use of accessories of transducers and cables other than those specified or sold by ackermann as replacement parts, may have as a consequence an increase of emission or decreased immunity of the medical... - Page 30 iNNOVATING TRADITION. ReCommenDeD sePARAtIon DIstAnCes The medical device is designed to be used in an electromagnetic environment in which interferences caused by RF radiation are controlled. The user or installer of the medical device can help prevent any electromagnetic interference by applying a minimum distance, according to the maximum power of the radio-frequency transmission equipment.
- Page 31 Ackermann User Manual 16 -2009 | Revision 01/20 by mAGnetIC AnD eleCtRomAGnetIC ImmunIty The medical device is designed for use in the magnetic and electromagnetic environment described in the table below. The user and/or installer must ensure conformity of the electromagnetic environment.
- Page 32 iNNOVATING TRADITION. eleCtRomAGnetIC ImmunIty, RADIo-FRequenCIes The medical device is designed for use in the magnetic and electromagnetic environment described in the table below. The user and/or installer must ensure conformity of the electromagnetic environment. electromagnetic Immunity test Test level Conformity level environment - guide CaUTION: RF portable communication devices must not be used (including peripheral devices such as antenna cables and external antennas) closer than 30 cm (12 inches) from any part of the medical device, including the cables specified by the manufacturer.
-
Page 33: Technical Specifications
- 1 USB 3.0 micro B Resolution: 1920 x 1080 p Recommended USB key format: FaT32 Definition: 1080 lines Compatible light cable types: ackermann, Storz® active pixel area: 1920 x 1080 and Olympus® Cable length 2.99 metres ... -
Page 34: Disposal And Recycling
innovAtInG tRADItIon. Chapter DIsPosAl AnD ReCyClInG This device bears a recycling symbol in accordance with european Directive 2002/96/eC concerning Waste electrical and electronic equipment (Weee). By correctly disposing of this device you will help prevent any harm to the environment and to human health. By correctly disposing of this device you will help prevent any harm to the environment and to human health. -
Page 35: Regulations And Standards
Ackermann User Manual 16 -2009 | Revision 01/20 by Chapter ReGulAtIons AnD stAnDARDs APPlICABle stAnDARDs AnD 11.1 This medical device complies with the essential requirements of european Directive 93/42/eC and european Regulation 2017/745. It was designed and manufactured in accordance with an eN ISO 13485-certified quality assurance system. - Page 36 innovAtInG tRADItIon. Symbol Description Complies with european Directive 93/42/eC and european Regulation 2017/745 Read user manual before use T UL/CSa time-delay fuses Potential equalization Video output ports and auxiliary control output port Protective ground (earth) Order number Serial number For medical devices, this symbol appears alongside the name and address of the year of manufacture. The latter is denoted by four figures.
- Page 37 Ackermann User Manual 16 -2009 | Revision 01/20 by Page: // 4 0...
- Page 38 iNNOVATING TRADITION. Page: // 4 0...
- Page 39 Ackermann User Manual 16 -2009 | Revision 01/20 by Page: // 4 0...
- Page 40 iNNOVATING TRADITION. www.ackermanninstrumente.de User Manual 16 -2009 | Revision 01/20 Page: // 4 0...






Need help?
Do you have a question about the 16-2009 and is the answer not in the manual?
Questions and answers