Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Kinetik TD3 Series
- Page 1 TD3 Series...
-
Page 2: Table Of Contents
Content Support Introduction 3 - 6 Indications for Use Safety Warning Contraindications Warnings 3 - 4 Precautions 4 - 6 Adverse Reactions Product Specifications Parts & Setup How to Use 9 - 10 Recommended Use Positions Cleaning and Maintenance Technical Information Troubleshooting 13 - 14 Environmental Condition for Transport and Storage... -
Page 3: Support
We promise to respond to all queries and will ensure to resolve any issue you may be having. You can reach us by… Phone: +44 1483 937969 Live Chat: Simply visit www.kinetikwellbeing.com and send us a message. Email: customercare@kinetikwellbeing.com Post: Kinetik Medical Devices Limited Unit 3, Perrywood Business Park, Honeycrock Lane, Salfords, Redhill. RH1 5DZ... -
Page 4: Introduction
Introduction Kinetik TENS Pain Reliever delivers electric impulses to tired and sore muscles. These different frequencies of impulses covering Transcutaneous Electrical Nerve Stimulation mimic the action potential coming from the central nervous system to trigger contraction of the muscle. It may be helpful in relieving aches and pains in various parts of the body such as the waist, shoulders, joints, hands and feet. -
Page 5: Precautions
into the chest may cause rhythm disturbances to the patient’s heart, which could be lethal. Do not apply stimulation over, or in proximity to, cancerous lesions. Do not apply stimulation when the patient is in the bath or shower. If you have one of the following conditions, please consult with your physician before purchasing or using this device. -
Page 6: Introduction
Do not apply stimulation of this device in the following conditions: (1) across the chest because the introduction of electrical current into the chest may cause rhythm disturbances to the heart, which could be lethal; (2) over painful areas. Please consult with your physician before using this device if you have painful areas;... -
Page 7: Precautions
(16) The device contains a lithium battery. If overheating of the device occurs while charging, stop the charging or operation immediately and report to Kinetik Wellbeing. (17) Dispose of the battery-containing device according to the local, state, or federal laws. -
Page 8: Adverse Reactions
Patients should stop using the device and should consult with their physicians if they experience adverse reactions from the device. Product Specifications Accessories included in the package. • Kinetik TD3 Tens Machine • 4 x Gel Pads • 2 x Output Cables • Micro USB Charger •... -
Page 9: Parts & Setup
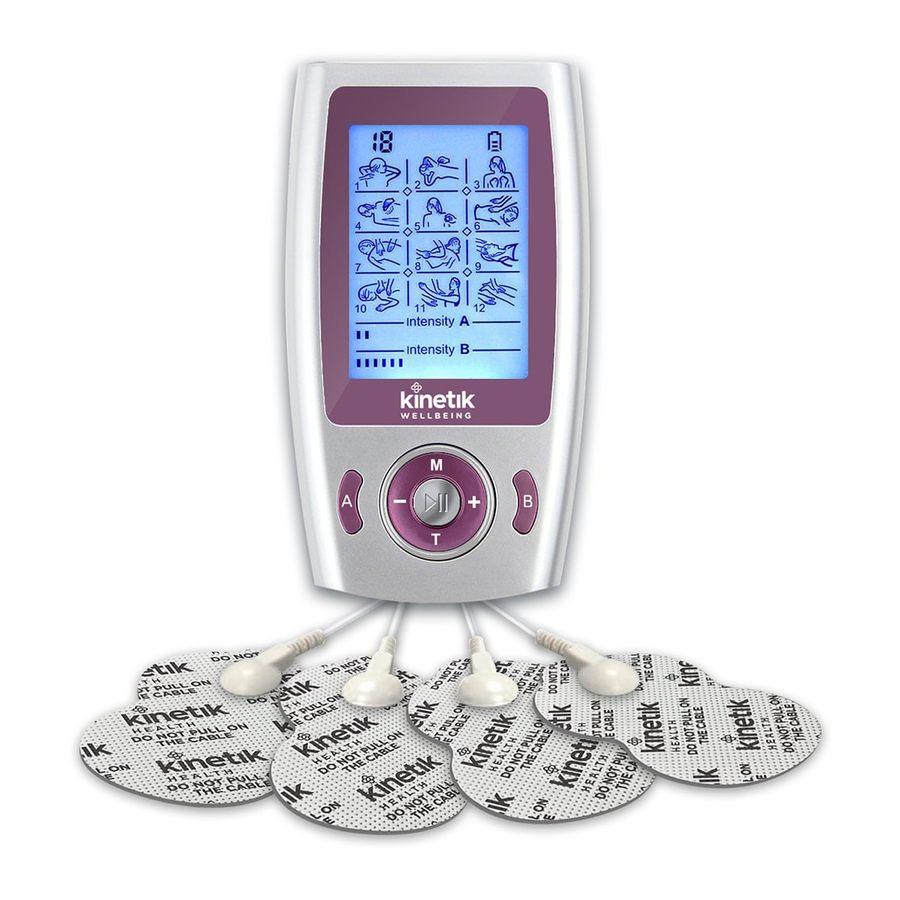
Parts & Setup Unpack the product, take the product and accessories out, charge device and then connect the electrode pad into the device. Then simply turn-on and select mode/intensity as desired. ON/OFF Countdown Timer Battery Indicator LCD Display Intensity Indicator Mode Selection Decrease Intensity Increase Intensity... -
Page 10: How To Use
How to Use • The Kinetik Wellbeing TD3 TENS Machine needs to be charged for up to 8 hours before the first use. • Connect a pair of electrode pads to one output cable – this is done by snapping them on. If required, connect the other pair of electrode pads to the remaining output cable. - Page 11 • Keeping the electrode pads in their plastic storage bags after use will extend lifespan. The electrode is disposable and should be replaced when it loses the adhesiveness. To purchase additional electrodes, please contact Kinetik Wellbeing. Product Programs Program name Time min.
-
Page 12: Recommended Use Positions
The electrode pads that come with the device are disposable and should be replaced after they lose their adhesiveness – please contact Kinetik Wellbeing for replacements. We recommend that users avoid the sticky side of the pads from touch. -
Page 13: Technical Information
Technical Information Model/type Weight Power supply Powered by internal 3.7V li-ion battery Automatic shutoff After 20 minutes without use Waveform and wave shape Biphasic rectangular wave pulse Degree of protection against electric shock Type BF applied part Pulse duration 100us (Microseconds) Type of protection against electric shock Internally powered equipment Pulse frequency... -
Page 14: Troubleshooting
Troubleshooting If your device is not operating properly, please check below for common problems and suggested solutions. If the recommended action does not solve the problem, please contact Kinetik Wellbeing. Problem Possible Cause Solution One pad feels stronger than the other This is normal. - Page 15 Troubleshooting Problem Possible Cause Solution No power source; no display on LCD The battery capacity is depleted Charge the battery The battery is weak Charge the battery Power cuts off during use The cord is broken Replace the cord Have you removed the transparent film from Peel off film on the adhesive surface of pads It is difficult to attach the pad to the the pad?
-
Page 16: Environmental Condition For Transport And Storage
Environmental condition for normal working, transport and storage - Normal working ambient temperature: 5~40°C - Normal working ambient humidity: 15~90% - Store and transport ambient temperature: -25 ~70°C - Store and transport ambient humidity: 0~90% - Atmospheric pressure: (70~ 106)kPa Symbols interpretation Fragile, handle with care Type BF applied part... -
Page 17: Safety Test Standards
Safety Test Standards • Medical Devices Directive 93/42/EEC • IEC 60601-1:2005+A1:2012/EN 60601-1:2006 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance • IEC60601-1-2 : 2014/EN60601-1-2 : 2014 Medical electrical equipment - Part 1-2: General require- ments for safety - Collateral standard: Electromagnetic compatibility - Requirements and tests •... -
Page 18: Emc And Fcc Statement
EMC and FCC statement Electromagnetic Compatibility and FCC Compliance Statement 1) This product needs special precautions regarding electromagnetic compatibility (EMC) and needs to be installed and put into service according to the EMC information provided, and this unit can be affected by portable and mobile radio frequency (RF) communications equipment. 2) Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. - Page 19 EMC and FCC statement Guidance and manufacture’s declaration – electromagnetic emission The device is intended for use in the electromagnetic environment specified below. The customer of the user of the device should assure that it is used in such an environment. Emission test Compliance Electromagnetic environment –...
- Page 20 EMC and FCC statement Guidance and manufacture’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance level...
- Page 21 EMC and FCC statement Guidance and manufacture’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance level...
- Page 22 EMC and FCC statement Guidance and manufacture’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance level...
- Page 23 EMC and FCC statement Guidance and manufacture’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance level...
- Page 24 EMC and FCC statement Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the device is used exceeds the applicable RF compliance level above, the device should be observed to verify normal operation.
- Page 25 EMC and FCC statement Recommended separation distances between portable and mobile RF communications equipment and the device. The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the device as recommended below, according to the maximum output power of the communications equipment.
-
Page 26: Emc And Fcc Statement
EMC and FCC statement The subject device has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. The product generates, uses, and can radiate radio frequency energy and, if not installed and used accordance with the instructions, may cause harmful interference to radio communications. -
Page 27: Contact Information
Contact Information Manufacturer : Harvard Medical Devices Ltd. HK Unit 1002, 10th Floor, Railway Plaza, 39 Chatham Road South, Tsimshatsui, Kowloon, Hong Kong. EC Authorized Representative : Share Info Consultant Service LLC Repräsentanzbüro Heerdter Lohweg 83, 40549 Düsseldorf TD3 IB UK 20190425 For support…...




Need help?
Do you have a question about the TD3 Series and is the answer not in the manual?
Questions and answers
How do I get the lot number for registration, it’s not on the box and I can’t see how you open it to access the batteries. Also this was a gift so I don’t have a receipt.