Summary of Contents for Welch Allyn ProBP 2000
- Page 1 Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Directions for use Software Version 1.X...
- Page 2 © 2018 Welch Allyn. All rights are reserved. To support the intended use of the product described in this publication. The purchaser of the product is permitted to copy this publication, for internal distribution only, from the media provided by Welch Allyn. No other use, reproduction, or distribution of this publication, or any part of it, is permitted without written permission from Welch Allyn.
-
Page 3: Table Of Contents
Contents Introduction ..................... 1 Intended use/Indications for use ................1 Contraindications ....................1 Symbols ....................... 2 About warnings and cautions ................5 Contents list ......................7 Controls and indicators ..................8 Power options ...................... 9 Screen elements ....................10 Insert or replace the batteries ................11 Position the blood pressure cuff on the patient .......... - Page 4 Contents Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 5: Introduction
Intended use/Indications for use The Welch Allyn ProBP 2000 Digital blood pressure device is intended for use in measuring blood pressure and heart rate in pediatric and adult patient populations 3+ years with arm circumferences between 15 cm to 55 cm (approximately 5.9 to 21.7 inches). -
Page 6: Symbols
Follow instructions/directions for use (DFU) -- mandatory action. A copy of the DFU is available on this website. A printed copy of the DFU can be ordered from Welch Allyn for delivery within 7 calendar days. Power symbols... - Page 7 Directions for use Introduction 3 Stacking limit by number Cuff symbols Artery marker Range Artery index marker Limb circumference (Minimum/Maximum) Lot Code Not made with natural rubber latex Miscellaneous symbols Authorized Representative in the European Community Manufacturer Date of manufacture Type BF applied part Serial Number...
- Page 8 4 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Product Identifier Lot Code Reorder Number Non-ionizing electromagnetic radiation Australian Communications and Media Authority (ACMA) Radio Compliance Mark (RCM) Global Trade Item Number Class II equipment IP22 Ingress protection: the device is protected against solid foreign objects of 12.
-
Page 9: About Warnings And Cautions
Directions for use Introduction 5 About warnings and cautions Warning and caution statements can appear on the Welch Allyn ProBP™ 2000 Digital Blood Pressure Device, the packaging, the shipping container, or in this Directions for use. Warnings and cautions WARNING Patient injury risk. The device is not suitable for measuring the blood pressure of neonatal infants or children. - Page 10 6 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device WARNING Inaccurate measurement risk. Do not place the cuff where it can disturb proper circulation. Do not place the cuff on any area where circulation is compromised or on any extremity used for intravenous infusions.
-
Page 11: Contents List
Directions for use Introduction 7 CAUTION Do not attempt to repair the unit yourself if it malfunctions. Only have repairs carried out by authorized service centers. CAUTION Report any unexpected operation or events to the manufacturer. CAUTION Use a soft cloth to clean the entire unit. Do not use any abrasive or volatile cleaners. -
Page 12: Controls And Indicators
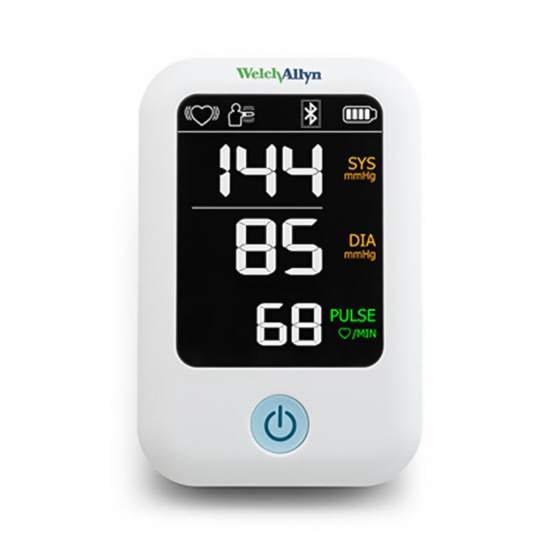
8 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Controls and indicators Device front No. Feature Description ® Apply to upper arm to take a blood pressure measurement FlexiPort blood pressure cuff Power button Powers on the blood pressure device and starts and stops a blood... -
Page 13: Power Options
Houses 4 AA alkaline batteries Power options CAUTION To get optimal performance and protect your device, use only the correct batteries or the Welch Allyn-approved power adapter. The device is powered by one of two sources: • 4 AA alkaline batteries •... -
Page 14: Screen Elements
10 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Screen elements The liquid crystal display (LCD) displays the following: systolic blood pressure (mmHg), diastolic blood pressure (mmHg), pulse rate (bpm), heart beat (regular or irregular) while acquiring blood pressure measurements, excessive motion alert, alarm priority, and battery charge level. -
Page 15: Insert Or Replace The Batteries
Directions for use Introduction 11 Symbol Description Motion indicator Motion may result in an inaccurate measurement. Reading out of range Either SYS > 260mmHg or DIA > 220mmHg. The symbol may appear in either the SYS or DIA area of the screen. Alarm priority = Low (an ! appears near the top of the screen) Reading out of range Either SYS <... -
Page 16: Position The Blood Pressure Cuff On The Patient
12 Introduction Welch Allyn ProBP™ 2000 Digital Blood Pressure Device 3. Replace the cover. Position the blood pressure cuff on the patient Before taking an NIBP measurement, follow these steps to properly attach the cuff to the patient. For information about obtaining blood pressure measurements, refer to Blood pressure guidelines at: https://www.welchallyn.com/probp2000.To achieve an... -
Page 17: Maintenance
This product and its components must be disposed of according to local laws and regulations. Do not dispose of this product as unsorted municipal waste. For more specific disposal or compliance information, see www.welchallyn.com/weee, or contact Welch Allyn Customer Service. -
Page 18: Troubleshooting
This section includes a list of error messages and frequently asked questions for problems you may encounter with your blood pressure device. If the device is not operating as you think it should, check here before contacting Welch Allyn Technical Support: http://www.welchallyn.com/support. - Page 19 Directions for use Maintenance 15 Physiological alarms Symptom Root cause Solution Out of range. Either SYS Power Press and hold the button. Measure >260mmHg or DIA >220mmHg. The again. If the problem persists, contact symbol may appear in either the www.welchallyn.com for further assistance.
- Page 20 16 Maintenance Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 21: Specifications
Specifications Item Specification Power supply: Battery powered 6VDC 4 AA batteries mode Power supply: AC adapter Input: 100–240V, 50–60Hz, 400mA Output: 6V, 1A powered mode Power supply model number UE08WCP-06100SPA Display mode Digital LCD V.A. 68mm x 90mm Measurement model Oscillometric testing mode Measurement range Rated cuff pressure: 0mmHg to 299mmHg (0kPa to 40kPa) - Page 22 Version 1.X Device lifetime The lifetime of the device is two (2) years. The manufacture date of the device can be found on the device label. Welch Allyn will service ProBP™ 2000 Digital Blood Pressure Devices that are within their lifetime.
-
Page 23: Transducer Accuracy Test
(2) a pressure meter simulator; ® (3) the device with the Flexiport connector removed. For further information or to order the test equipment, contact Welch Allyn Technical Support at: http:// www.welchallyn.com/support. Item Test volume repair fixture... - Page 24 20 Specifications Welch Allyn ProBP™ 2000 Digital Blood Pressure Device 2. Set up the test equipment. a. Connect the device tubing to the 4-way tee. b. Connect the silicone rubber tubing to the 4-way tee and to the 500 ml volume port of the test manifold.
-
Page 25: Complied Standards List
Directions for use Specifications 21 12. After the test completes, disassemble the test equipment and slide the end of the ® device tubing over the Flexiport hose fitting barb. 13. Open the battery door and remove one of the batteries to power off the device. Note Press the Power button to ensure that all power has been removed from the device. - Page 26 22 Specifications Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 27: General Radio Compliance
General radio compliance The wireless features of this device must be used in strict accordance with the manufacturer’s instructions as described in the user documentation that comes with the product. This device complies with Part 15 of the FCC rules and with the rules of the Canadian ICES-003 as described below. -
Page 28: Industry Canada (Ic) Compliance
24 General radio compliance Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Industry Canada (IC) compliance To ensure compliance with FCC and Industry Canada RF exposure requirements, this device must be installed in a location where the antennas of the device will have a minimum distance of at least 20 cm from all persons. - Page 29 Directions for use General radio compliance 25 French Par conséquent, Guangdong Transtek Medical Electronics Co., Ltd. déclare que cet émetteur de faible puissance est conforme aux exigences essentielles et autres dispositions pertinentes de la directive 2014/53/CE German Hiermit erklärt Grason-Stadler, dass dieser Niedrigleistungssender den grundlegenden Anforderungen und anderen relevanten Bestimmungen der Richtlinie 2014/53/EG entspricht Greek Με...
- Page 30 26 General radio compliance Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 31: Warranty
Warranty Welch Allyn will warranty the blood pressure device to be free of defects in material and workmanship and to perform in accordance with manufacturer specifications for the period of one year from the date of purchase from Welch Allyn or its authorized distributors or agents. - Page 32 28 Warranty Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 33: Approved Accessories
Approved accessories Item Description REUSE-11L Adult long cuff (25–34cm) 107041 RPM BP AC Adapter. This adapter is an alternate power source for the blood pressure device. For a list of additional cuff sizes visit www.welchallyn.com/probp2000. - Page 34 30 Approved accessories Welch Allyn ProBP™ 2000 Digital Blood Pressure Device...
-
Page 35: Emc Guidance And Manufacturer's Declarations
EMC guidance and manufacturer’s declarations EMC guidance 1. This product needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided, and this unit can be affected by portable and mobile RF communications equipment. 2. -
Page 36: Emissions And Immunity Information
32 EMC guidance and manufacturer’s declarations Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Emissions and immunity information Electromagnetic emissions The ProBP™ 2000 Digital Blood Pressure Device is intended for use in the electromagnetic environment specified below. The customer or user of the ProBP™ 2000 Digital Blood Pressure Device should assure that it is used in such an environment. - Page 37 Directions for use EMC guidance and manufacturer’s declarations 33 Guidance and manufacturer’s declaration – electromagnetic immunity IEC 61000-4-11 0% 1 cycle and 70% 0% 1 cycle and 70% 25/30 cycles 25/30 cycles Single phase: at 0 Single phase: at 0 0% 300 cycle 0% 300 cycle Power frequency...
- Page 38 34 EMC guidance and manufacturer’s declarations Welch Allyn ProBP™ 2000 Digital Blood Pressure Device Guidance and manufacturer’s declaration –electromagnetic immunity If the measured field strength in the location in which the ProBP™ 2000 Digital Blood Pressure Device is used exceeds the applicable RF compliance level above, the ProBP™...
- Page 39 Directions for use EMC guidance and manufacturer’s declarations 35 Guidance and manufacturer’s declaration –electromagnetic immunity 380-390 GMRS 460, FM c) ± 5kHz FRS 460 deviation 1kHz sine Pulse LTE Band modulation b) 704-787 13, 17 217Hz 800-960 GSM 800/ Pulse 900, TETRA modulation b) 800 iDEN...














Need help?
Do you have a question about the ProBP 2000 and is the answer not in the manual?
Questions and answers
How do you get the cuff to deflated
To deflate the cuff on the Welch Allyn ProBP 2000, open the cuff to release the air and then loosen and remove it from the patient's arm.
This answer is automatically generated