Summary of Contents for Boston Scientific Swiss LithoCast Trilogy FT-235
- Page 1 INSTRUCTIONS FOR USE Caution! Federal (USA) law restricts this device FT-235 to sale by or on the order of a physician...
- Page 3 The product must be used by qualified operating room support in case of technical problems. Please contact personnel (with extensive training in urology) in hospitals, your local Boston Scientific sales representatives. clinics and medical universities to treat affected patients We wish you lots of success! of any age.
-
Page 4: Table Of Contents
CONTENTS 6.6. CONSERVING THE STONE CATCHER 1. WARNING CONTENTS 2. COMPONENTS 6.7. DISPOSING OF SINGLE-USE COMPONENTS 3. INSTALLATION 6.8. SWITCHING OFF THE CONSOLE 3.1. INSTALLING THE CONSOLE 7. CLEANING, DISINFECTING, 3.2. FILLING THE COOLING SYSTEM AND STERILIZING 3.3. CONNECTING THE CONSOLE TO THE 7.1. - Page 5 1. WARNING Boston Scientific (distributor) and EMS accept no liability for direct or consequential injury or damage resulting from improper use, arising in particular through non-observance of the operating instructions, or improper preparation and maintenance. Before using this product, please carefully read,...
-
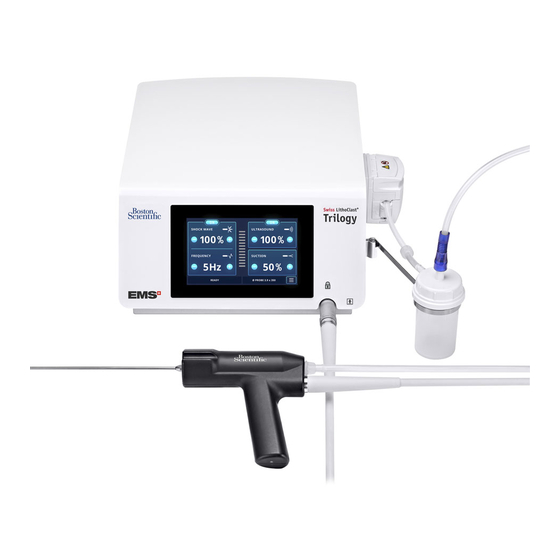
Page 6: Components
2. COMPONENTS The components provided for your device will vary, according to your configuration. NON STERILE ZONE DESIGNATION Console (with peristaltic pump) or Cart - optional Fluid management system - optional USB key 2.5 L Demineralized water Stone catcher support Cooling system filling kit Power cord Wired pedal... - Page 7 STERILE ZONE DESIGNATION STERILE STATE Stone catcher - optional Provided sterile Multiuse torque wrench To be sterilized before use Probe Provided sterile Unclogging rod To be sterilized before use Aspiration plug To be sterilized before use Handpiece To be sterilized before use Figure 2 DESIGNATION TO BE USED...
-
Page 8: Installation
3. INSTALLATION Please make sure that you have all the required parts 3.2. FILLING THE COOLING SYSTEM and tools to complete the installation of your device prior to starting work To avoid interruptions during treatment, make sure that the cooling liquid is above the minimum level Refer to the Packing List. - Page 9 2. Fill the filling bottle and close it. 5. Push the filling tube into the filling inlet connector until it engages. Figure 8 Figure 11 6. Invert the filling bottle and squeeze it to fill the tank. Only use demineralized water to fill the cooling system.
-
Page 10: Connecting The Console To The Equipotential Conductor
7. Push the metal locking part down to remove the filling 3.3. CONNECTING THE CONSOLE TO THE tube. EQUIPOTENTIAL CONDUCTOR When applicable and according to your in-house protocol, connect the equipotential conductor at the rear of the console with the bus bar. The equipotential conductor provides a connection between the unit and the potential equalization bus bar of the electrical installation when necessary. -
Page 11: Installing The Pedal
3.5. INSTALLING THE PEDAL 3.6. INSTALLING THE STONE CATCHER 1. Connect the pedal cord to the corresponding connector Case 1: Use of a sterile, single-use Stone Catcher at the rear of the console. (optional) Pay attention to the pedal cord connector indexa- 1. - Page 12 4. Open the pump. Case 2: Use of an in-house aspiration system. 1. Screw the aspiration plug to the handpiece. Figure 23 5. Place the stone catcher output tube into the pump. Figure 26 2. Connect the in-house aspiration system on the aspiration plug.
-
Page 13: Installing The Single-Use Fluid Management System Set (Optional) And Replacement Pouch
4. When the open pouch is filled, open the closed clamp 3.7. INSTALLING THE SINGLE-USE FLUID (B) first. MANAGEMENT SYSTEM SET (OPTIONAL) AND REPLACEMENT POUCH 5. Close the open clamp (C) (adjacent to the filled pouch). 6. The filled pouch can be exchanged for a new empty pouch, using the Luer-lock connection. -
Page 14: Installing A Probe On The Handpiece
2. Remove the protective cap from the console. 2. Use the wrench to firmly tighten the appropriate probe on the handpiece. Multiuse Torque wrench Figure 32 3. Connect the handpiece to the console. Figure 34 3.10. CONNECTING THE POWER CORD Connect only to a FI protected mains power supply (FI = Residual current protection). -
Page 15: Getting Started
4. GETTING STARTED 3. The console automatically performs a series of 4.1. STARTING THE DEVICE diagnostic tests. 1. Use the mains power switch located on the rear panel 4. The console displays a green check mark for each to switch on the console. successfully completed diagnostic test. - Page 16 Click this pictogram Meaning Action Log file To download the log file and save it on a USB drive. download Several screens will appear. Choose a To select the display language. language Refer to the Setting the Language section. Brightness Use the buttons to adjust the display brightness.
-
Page 17: Equipment Data
2. Click the language you want to select. 2. Select Console to view the installed software version number, product serial number, and cumulated treatment statistics. Figure 41 3. To confirm the selected language, click OK. Figure 44 3. Select Handpiece to view the handpiece serial number and cumulated treatment statistics. -
Page 18: Treatment
5. TREATMENT Do not let the handpiece remain in contact with the 5. Press the pedal completely (STEP 2) to activate both patient during treatment. suction and energies and make sure that the quality meter is in the green zone and the fluid is moving During treatment, an auditory information pulse will through the suction tube. -
Page 19: Probe Insertion
3. All probe and handpiece usage information are 5.2. PROBE INSERTION automatically recorded in the console (number of uses, time of use, etc.). Do not start treatment without ensuring that a back-up probe is available. 4. According to the type of treatment, two pre-settings are available: To avoid bending the probe, make sure that the probe and the endoscope are aligned. - Page 20 2. If required, adjust any settings manually as described in the following table: PICTOGRAMS MEANING ACTION Use the ON/OFF button to activate or deactivate the functionality in ON/OFF button question. Use the buttons to adjust the impact power in percent Impact power from 10% to 100% (in 10% increments).
-
Page 21: Adapting Suction Flow Rate
5.4. ADAPTING SUCTION FLOW RATE 5.5. STARTING TREATMENT To adapt the suction flow rate: 1. Go to the READY screen to start the treatment. 1. Use the suction flow rate control as described 2. Press the pedal halfway (STEP 1) to activate the in Table 3. -
Page 22: Post-Treatment Procedure
6. POST-TREATMENT PROCEDURE 6.1. COMPLETING TREATMENT 1. Remove the probe from the endoscope. Figure 53 Figure 55 Do not disconnect the probe and the handpiece To accelerate the emptying procedure, the stone at this stage. catcher can be disconnected from the handpiece. 2. -
Page 23: Disconnecting The Handpiece
Figure 59 Figure 57 1. Pull back the metallic part of the handpiece connector to disconnect the handpiece. Figure 60 If the mechanical disconnection of the handpiece is not possible when the console is switched off, Figure 58 refer to the Troubleshooting section. 2. -
Page 24: Recording Treatment Data
6.3. RECORDING TREATMENT DATA 1. Select History to view the statistics for the last 5 treatment sessions. From the READY screen: From the STAND BY screen: ð Table 4 2. Information on the previous treatment sessions will be displayed. Figure 62 3. -
Page 25: Disconnecting The Stone Catcher
6.4. DISCONNECTING THE STONE CATCHER 6.8. SWITCHING OFF THE CONSOLE 1. Disconnect the stone catcher from the handpiece Make sure that the lock icon is switched off and from the fluid management system or from your before turning off the console. vacuum system. -
Page 26: Cleaning, Disinfecting, And Sterilizing
7. CLEANING, DISINFECTING, AND STERILIZING Cleaning 7.1. MULTIUSE COMPONENTS EMS recommends using Neodisher® MediClean as the Step A: Preparation at the Point of Use cleaning agent as it has been used for the validation After contamination, the sample is allowed to dry study. - Page 27 Step C2. Automated Cleaning, disinfection and drying Step E. Packaging for sterilization process Prior to sterilization, the products must be placed in a Automated Cleaning, disinfection and drying validation suitable sterilization container or sterilization packaging: has been performed using a Miele 7735CD washing Compliant with EN ISO 11607 or EN 868.
-
Page 28: Console, Pedal, And Cart
5. Use a cleaning wipe with proven efficacy (e.g., enzol 7.2. CONSOLE, PEDAL, AND CART 2%) to clean the surfaces. 1. Turn off the console. The housing of the console is not waterproof. 6. To disinfect use 70% isopropyl alcohol or other EPA-recognized surface disinfectant. -
Page 29: Product Maintenance
For the spare parts described below, please refer to the order form or contact local Boston Scientific sales representative. 8.1. COOLING LIQUID CIRCUIT MAINTENANCE The cooling liquid and the water filter must be replaced every year. -
Page 30: Replacing Fuses
7. Replace the console on a flat surface. 8.3. DOWNLOADING LOGFILE 8. Refill the cooling system. Refer to the Filling the Your local Boston Scientific sales representatives may Cooling System section. request this procedure. 1. Plug the USB key provided by EMS at the rear of the console. -
Page 31: Product Storage And Shipping
9. PRODUCT STORAGE AND SHIPPING Do not tilt or invert the console without first having 5. Put the draining tube in a receptacle that is more than emptied the cooling liquid circuit. 600 ml in volume. Always empty the cooling liquid circuit before longterm storage (2 weeks or more) or shipping to avoid damage to the console. -
Page 32: Shipping The Product
8. Unlock the metal locking part to disconnect the 9.2. SHIPPING THE PRODUCT draining tube. Before shipping the product, follow the instructions provided in the Cleaning, Disinfecting and Steril- izing section. To avoid damage, pack the product and all acces- sories in the original packaging. -
Page 33: Product Disposal
Keep the original packaging until the product is to be disposed of permanently. 11. TECHNICAL SUPPORT Please contact your local Boston Scientific sales repre- sentative for any product servicing or repairs. Boston Scientific and EMS declines responsibility for the safety of the product and declares the warranty null and void if service or repair is carried out by an unauthorized third party or if non-genuine spare parts are used. -
Page 34: Troubleshooting
12. TROUBLESHOOTING Ensure that the product and the accessories have 12.2. WEAK SUCTION been used in accordance with the conditions specified by specified in the instructions for use. 1. Make sure that the stone catcher tube is correctly inserted in the peristaltic pump. To improve our quality of service, please provide the following information: 2. - Page 35 1. Press the highlighted faulty component and follow 3. If the solutions proposed fail to solve the problem, the interactive menu to identify the exact origin of please contact your local Boston Scientific sales repre- the error. sentatives. Do not, in any case, return a product before troubleshooting of the error has been performed.
- Page 36 E025 - Console temperature error. Please wait for the console to cool down. Please contact your service center if the error persists. E026 - Shockwave module critical error. Please restart the device. Please contact your service center if the error persists.
-
Page 37: New Electromagnetic Compatibility
13.ELECTROMAGNETIC COMPATIBILITY The SWISS LITHOCLAST® TRILOGY should not be used adjacent to or stacked with another SWISS LITHOCLAST® TRILOGY. If adjacent or stacked use is necessary, the SWISS LITHOCLAST® TRILOGY should be observed to verify normal operation in the configuration in which it will be used. Portable and mobile RF communications equipment should be used no closer than 30 cm to any part of the SWISS LITHOCLAST®... - Page 38 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The SWISS LITHOCLAST® TRILOGY is intended for use in the electromagnetic environment specified below. The customer or the user of the SWISS LITHOCLAST® TRILOGY should ensure that it is used in such an environment. IEC 60601 Electromagnetic Immunity test...
- Page 39 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The SWISS LITHOCLAST® TRILOGY is intended for use in the electromagnetic environment specified below. The customer or the user of the SWISS LITHOCLAST® TRILOGY should ensure that it is used in such an environment. Portable and mobile RF communications equipment should be used no closer to any part of the SWISS LITHOCLAST®...
- Page 40 Proximity fields 27 V/m 27 V/m wireless equipment from RF wireless 380-390 MHz 380-390 MHz maximum output power and communications 50% PM 18 Hz 50% PM 18 Hz separation distance tested (at equipment 30 cm): IEC 61000-4-3 28 V/m 28 V/m 430-470 MHz 430-470 MHz TETRA 400: max 1.8 W...
- Page 41 3.2 m 3.2 m 3.2 m 10 m 10 m 10 m Table 11 For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where power (P) is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
-
Page 42: Technical Data
14. TECHNICAL DATA MANUFACTURER E.M.S. Electro Medical Systems S.A., CH-1260 Nyon, Switzerland MODEL Swiss LithoClast® Trilogy POWER SUPPLY 100 – 240 VAC, 50 – 60 Hz, 500 VA OUTPUT POWER (ULTRASOUND) 70 Watt OUTPUT POWER (SHOCK) 80 Watt EN 60601-1 CLASSIFICATION System: EN 60601-1: Class I Probe: EN 60601-1: Class I BF MDD 93/42 EEC CLASSIFICATION... -
Page 43: Symbols
15. SYMBOLS Distributor logo Manufacturer logo Product name Origin of the product Prescription device Global Trade Item Number Non Sterile CE marking CSA marking with “C” identification for products in conformance with Canadian standards and “US” for products in conformance with US standards Batch Number DEKRA INMETRO identification for products in conformance with Brazilian electrical standards... - Page 44 Manufacturer Date of manufacture Catalogue number Disposal of Old Electrical & Electronic Equipment (Applicable in the European Union and other European countries with separate collection systems) Equipotential plug Serial number Refer to the instruction manual Device requiring protective earth Input Fuse Risk of electric shock Emptying...
- Page 45 Minimum tank level indicator Maximum tank level indicator Degree of protection against water permeability USB connector HDMI connector Thermal disinfection 135°C Sterilizable at up to 135°C in the autoclave Do not re-sterilize STERILIZE Do not re-use Do not use if package is damaged Refer to instruction manual Content STERILE...
-
Page 46: Appendix
16. APPENDIX 16.1. PROBE COMPATIBILITY TABLE Different probe sizes are available to allow effective treatment with the most popular endoscopic systems for percu- taneous nephroscopy, rigid and semi-rigid ureteroscopy and cystoscopy: PROBE DIAMETER MINIMUM MAXIMUM TAG RING AND LENGTH ENDOSCOPE ENDOSCOPE COLOR WORKING CHANNEL... - Page 47 IC Statements: This device complies with Industry Canada license-exempt RSS standard(s). Operation is subject to the following two conditions: (1) this device may not cause interference, and (2) this device must accept any interference, including interference that may cause undesired operation of the device. Under Industry Canada regulations, the radio transmitter(s) in this device may only operate using an antenna of a type and maximum (or lesser) gain approved for the transmitter by Industry Canada.
- Page 48 D i st r i bute d by: B o s ton S cient ific Cor p o ra t i o n 3 00 B oston S cie nt i fi c Wa y M a rl b orough , M A 01 7 5 2 Cu s to mer S e r vice 1- 8 8 8- 2 7 2 - 1 0 0 1 M a nufa c t ure d by : E MS Ele c tro M ed ical Sys te m s SA...















Need help?
Do you have a question about the Swiss LithoCast Trilogy FT-235 and is the answer not in the manual?
Questions and answers