Summary of Contents for BIOTRONIK Cardiac Airbag
- Page 1 (Draft) Cardiac Airbag / Cardiac Airbag-T Family of Implantable Cardioverter Defibrillators and Software Cartridge for PLUS PLUS TMS 1000 and EPR 1000 Technical Manual...
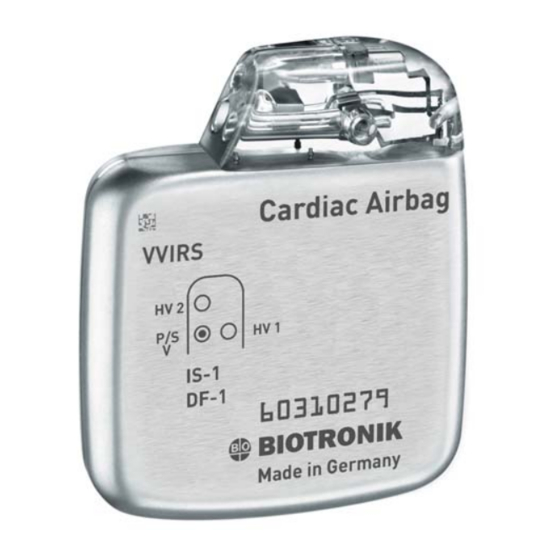
- Page 2 Cardiac Airbag/Cardiac Airbag-T Implantable Cardioverter Defibrillator Inside the housing, top left-hand side: Year of manufacture X-Ray identification AUTION Federal (U.S.A.) law restricts this device to sale by, or on the order of, a physician. 2003 BIOTRONIK, Inc., all rights reserved.
-
Page 3: Table Of Contents
Cardiac Airbag Technical Manual i Contents 1. General................1 1.1 System Description............1 1.2 Indications and Usage ..........2 1.3 Contraindications ............2 1.4 Warnings and Precautions.........3 1.4.1 Sterilization, Storage, and Handling ....3 1.4.2 Device Implantation and Programming....4 1.4.3 Lead Evaluation and Connection.......6 1.4.4 Follow-up Testing..........7... - Page 4 Pulse Amplitude ..........32 2.5.8 Pulse Width ............33 2.5.9 Noise Response..........33 2.5.10 Post Shock Pacing..........33 2.6 Special Features ............33 2.6.1 Home Monitoring (Cardiac Airbag-T Only) ..33 2.6.2 Real-time IEGM Transmission......37 2.6.3 Capacitor Reformation ........37 2.6.4 Patient and Implant Data ........38 2.6.5 System Status ............39...
- Page 5 Cardiac Airbag Technical Manual iii 5.6 Blind Plug Connection ..........55 5.7 Program the ICD............56 5.8 Implant the ICD ............56 5.9 Suggested Cardiac Airbag Implant Procedure ..57 6. Follow-up Procedures ..........68 6.1 General Considerations ..........68 6.2 Suggested Cardiac Airbag Follow-Up Procedure..68 6.3 Longevity..............74...
- Page 6 Cardiac Airbag Technical Manual Cardiac Airbag Specifications Battery Voltage: 6.3 Volts Maximum Shock Energy: 30 joules Defibrillation Lead Ports Two DF-1 (3.2 mm) Pacing Lead Ports One IS-1 (3.2 mm) Dimension: 55 x 67 x 13 mm Volume: 39 cc...
-
Page 7: General
The ICDs are designed to collect diagnostic data to aid the physician’s assessment of a patient’s condition and the performance of the implanted device. The Cardiac Airbag ICDs are specifically designed to have reduced complexity for implant and follow-up, yet provide essential therapies for conversion of life threatening ventricular tachyarrhythmias. -
Page 8: Indications And Usage
2 Cardiac Airbag Technical Manual The Cardiac Airbag and Cardiac Airbag-T have two DF-1 defibrillation / cardioversion and one IS-1 pacing/sensing header ports. IS-1 refers to the international standard whereby leads and generators from different manufacturers are assured a basic fit [Reference ISO 5841-3:1992]. -
Page 9: Warnings And Precautions
Cardiac Airbag Technical Manual 3 1.4 Warnings and Precautions ATP (Anti-Tachycardia Pacing) – The Cardiac Airbag ICD does not provide ATP therapy. Do not implant this ICD in patients with documented ventricular tachycardias unless high energy defibrillation is desired for treatment of the ventricular arrhythmia. -
Page 10: Device Implantation And Programming
4 Cardiac Airbag Technical Manual Storage (temperature) - Store the device between 5° to 55°C (41° - 131° F) because temperatures outside this range could damage the device. Storage (magnets) - To avoid damage to the device, store the device in a clean area, away from magnets, kits containing magnets, and sources of electromagnetic interference (EMI). - Page 11 (or explanted if previously implanted). Programmed Parameters – Program the device parameters to appropriate values based on the patient’s specific arrhythmias and condition. Programmers - Use only BIOTRONIK programmers to PLUS communicate with device (TMS 1000...
-
Page 12: Lead Evaluation And Connection
6 Cardiac Airbag Technical Manual Shock Impedance - If the shock impedance is less than twenty- five ohms, reposition the lead system to allow a greater distance between the electrodes. Never implant the device with a lead system that has measured shock impedance as less than twenty-five ohms. -
Page 13: Follow-Up Testing
Tightening Setscrew(s) – Do not overtighten the setscrew(s). Use only the BIOTRONIK supplied torque wrench. Sealing System – Be sure to properly insert the torque wrench into the perforation at an angle perpendicular to the connector receptacle. -
Page 14: Pulse Generator Explant And Disposal
8 Cardiac Airbag Technical Manual Resuscitation Availability - Ensure that an external defibrillator and medical personnel skilled in cardiopulmonary resuscitation (CPR) are present during post-implant device testing should the patient require external rescue. Safe Program – Within the EP Test screen, pressing the “Safe Program”... -
Page 15: Hospital And Medical Hazards
Cardiac Airbag Technical Manual 9 1.4.6 Hospital and Medical Hazards Electromagnetic interference (EMI) signals present in hospital and medical environments may affect the function of any ICD or pacemaker. The ICD is designed to selectively filter out EMI noise. However, due to the variety of EMI signals, absolute protection from EMI is not poss ible with this or any other ICD. - Page 16 10 Cardiac Airbag Technical Manual External Defibrillation - The device is protected against energy normally encountered from external defibrillation. However, any implanted device may be damaged by external defibrillation procedures. In addition, external defibrillation may also result in permanent myocardial damage at the electrode-tissue interface as well as temporary or permanent elevated pacing thresholds.
-
Page 17: Home And Occupational Hazards
6 inches (15 centimeters) of the ICD, when the ICD is programmed to standard sensitivity. Patients having an implanted BIOTRONIK ICD who operate a cellular telephone should: •... -
Page 18: Electronic Article Surveillance (Eas)
12 Cardiac Airbag Technical Manual Based on results to date, adverse effects resulting from interactions between cellular telephones and implanted ICDs have been transitory. The potential adverse effects could include inhibition or delivery of additional therapies. electromagnetic interference (EMI) emitting from a telephone... -
Page 19: Adverse Events
Cardiac Airbag Technical Manual 13 1.5 Adverse Events 1.5.1 Potential Adverse Events The following is a list of the potential risks that may occur with this device: • Acceleration of arrhythmias • Air embolism • Bleeding • Chronic nerve damage •... -
Page 20: Observed Adverse Events
Phylax XM and Phylax 06 ICDs, which are earlier versions of the Cardiac Airbag ICDs. The observed adverse events are applicable because the Cardiac Airbag ICD is a downsized version of the Phylax XM with rate adaptive pacing capabilities. - Page 21 Cardiac Airbag Technical Manual 15 Two ICDs were explanted during the trial. One was secondary to the patient being unable to tolerate further testing required by the clinical protocol. The other was secondary to a systemic infection; the patient was subsequently implanted with another device.
- Page 22 16 Cardiac Airbag Technical Manual Event # of pts % of # of with with High DFT’s 0.6% 0.02 Minor stroke 0.6% 0.01 Renal failure 0.6% 0.01 Required additional drug 0.6% 0.01 therapy ICD/lead connection 0.6% 0.01 ICD therapy during lead 0.6%...
-
Page 23: Clinical Studies
Airbag-T ICD is based on other US distributed BIOTRONIK products. Due to the similarities between the Cardiac Airbag / Cardiac Airbag-T, Belos VR / VR-T, and Phylax XM and the limited nature of these changes, a clinical study of the Cardiac Airbag / Cardiac Airbag-T ICD was determined to be unnecessary. -
Page 24: Methods
18 Cardiac Airbag Technical Manual 1.6.2 Methods The multicenter clinical investigation was designed to validate the safety and effectiveness of the ICD system to detect and treat monomorphic ventricular tachycardia (MVT), polymorphic ventricular tachycardia (PVT), ventricular fibrillation (VF), and bradycardia. - Page 25 Cardiac Airbag Technical Manual 19 Table 2: Clinical Study Results Description Study Group [95% CI] Tachyarrhythmia Conversion Rate 1 95.8% (496/518) [93.6%, 97.3%] Induced Spontaneous 99.7% (1540/1544) [99.3%, 99.9%] Total 98.7% (2036/2062) [98.2%, 99.2%] Sudden Cardiac Death Survival 100.0% (39/39) (at one year) [91.0%, 100.0%]...
-
Page 26: Patient Selection And Treatment
SVTs can initiate unwanted device therapy. • Direct any questions regarding individualization of patient therapy to your BIOTRONIK representative or BIOTRONIK technical services at 1-800-547-0394. 1.7.2 Specific Patient Populations Pregnancy - If there is a need to image the device, care should be taken to minimize radiation exposure to the fetus and the mother. -
Page 27: Evaluating Prospective Icd Patients
(For additional copies of the patient manual, contact the BIOTRONIK at the address listed in this manual.) 1.9 Evaluating Prospective ICD Patients The prospective ICD implant candidate should undergo a cardiac evaluation to classify any and all tachyarrhythmias. -
Page 28: Device Features
22 Cardiac Airbag Technical Manual 2. Device Features The Cardiac Airbag family feature set is presented under the following sub-headings: Sensing, Tachyarrhythmia Detection, Tachyarrhythmia Redetection, Tachyarrhythmia Therapy, Bradycardia Therapy, and Special Features. The features apply to all members of the Cardiac Airbag family except where specifically referenced differently. - Page 29 Cardiac Airbag Technical Manual 23 Table 3: Sensitivity Settings Setting Definition for Use Standard This setting is recommended for most patients, especially for those with measured R-wave amplitude of ≥3 mV. Enhanced This setting offers suppression of T-wave T Wave oversensing.
- Page 30 24 Cardiac Airbag Technical Manual Figure 1. Automat ic Sensitivity Control with Standard Setting Figure 1 provides a n illustration of Automatic Sensitivity Con trol with the sensitivity programmed to Standard. The tracked R – ave is measured to be 6.0 mV following the sensed refractory period the UT is set to 3.0 mV.
-
Page 31: Minimum Ventricular Threshold
0.8 mV, but it can be adjusted from 0.5 to 2.5 mV. 2.2 V entricular Tachyarrhythmia Detection The Cardiac Airbag ICDs detect and measure the rate of sensed cardiac signals to discriminate ventricular tachyarrhythmias from sinus rhythm or sinus bradycardia. -
Page 32: Vf Classifications
VT zone is designed as a “Mon itoring Zone” only and no therapies are available. In addition, when the Cardiac Airbag senses the programmed number of consecutive intervals (termination count) within the sinus rate zone, all tachyarrhythmia detection criteria, including... -
Page 33: Onset And Stability
Cardiac Airbag Technical Manual 27 2.2.4 Onset and Stability In addition to the standard tachycardia detection parameters previously described, the VT Monitoring Zone incorporates two additional detection enhancements: Onset and Stability. Both Onset and Stability are preset to standard values and are not programmable for the VT Monitoring Zone. -
Page 34: Tachyarrhythmia Redetection
Termination of a ventricular tachyarrhythmia episode is declared when 12 out of 16 consecutive sensed intervals are l onger than the VT-interval counter. 2.4 Tachyarrhythmia Therapy The Cardiac Airbag ICDs offers only defibrillation therapy for the treatment of ventricular tachyarrhythmias classified as VF. -
Page 35: Shock Therapy
Cardiac Airbag Technical Manual 29 2.4.1 Shock Therapy The Cardiac Airbag ICDs offer shock therapy only for the VF rate classifications. Up to 8 shocks are available for the VF zone for each episode detected. The first defibrillation shock in the therapy sequence is delivered with confirmation (while the capacitors are being charged). - Page 36 100% Figure 2. Biphasic Waveform 2.4.1.4 Shock Energy The Cardiac Airbag ICDs are designed to ensure that the energy programmed for therapy is the same as what is actually delivered to the patient regardless of the lead impedance. 2.4.1.5 Shock Polarity The polarity of the shock therapy is non-programmable and preset to Normal.
-
Page 37: Bradycardia Therapy
2.5.3 Rate Adapt ation Cardiac Airbag / Cardiac Airbag-T ICDs allow the selection of a rate responsive pacing mode (VVIR). This mode allows the ICDs bradycardia therapy function to adapt the pacing rate to increasing or decreasing patient activity, based on data collected from a motion sensor within the ICD. -
Page 38: Gain And Threshold
32 Cardiac Airbag Technical Manual 2.5.4 Gain and Threshold The Gain defines how much the sensor signal is amplified before it is used by the rate adaptive algorithm. The Gain is programmed so the maximum desired pacing rate during exercise occurs at a maximum exertion level. The Gain is preset to 4 when programmed to the VVIR mode. -
Page 39: Pulse Width
The pulse width is independently set for normal and post-shock bradycardia pacing. 2.5.9 Noise Response The Cardiac Airbag ICD’s response to detected noise is to deliver asynchronous pacing in ventricular channel. 2.5.10 Post Shock Pacing Separately, bradycardia pacing support is available with the ICD following shock therapy delivery. - Page 40 34 Cardiac Airbag Technical Manual 2.6.1.1 Transmission of Information The implant transmits information with a small transmitter, which has a range of about 2 meters. The transmissions are activated by the detection of an arrhythmia episode, as programmed. The types of transmissions are discus sed in Section 2.6.1.4...
- Page 41 BIOTRONIK Service Center and processed into a concise report called a Cardio Report. The Cardio Report is titled depending on the type of event transmission. This Cardio Report contains current and previous implant data. The Cardio Report is sent to the attending physician via fax.
- Page 42 36 Cardiac Airbag Technical Manual To ensure successful transmission of the patient data, Cardiac Airbag-T is programmed to send up to 10 repetitive transmissions of identical data at an hourly time interval. Event Report - When certain cardiac and technical...
-
Page 43: Real-Time Iegm Transmission
Cardiac Airbag Technical Manual 37 Leads • Pace impedance (ventricular) • Shock impeda • Date of impedance measurements Device Status Summary • Status • Remarks 2.6.2 Real-time IEGM Transm ission The pulse generators provide real time transmission of the unfi lter d intracardiac electrogram (I EGM) to the programmer. -
Page 44: Patient And Implant Data
38 Cardiac Airbag Technical Manual An automatic or manually initiated capacitor reform fully charges the capacitors and then allows the capacitors to drain off through the internal circuitry of the ICD. No shock will be delivered to the pati ent. Throughout the reform... -
Page 45: System Status
4) Displayed data includes ICD information, charge circuit parameters, capacitor reformation data, battery status, and lead information. The system status screen presents a large variety of information about the Cardiac Airbag ICDs including: • Serial number (always displayed after interrogation) •... -
Page 46: Holter Memory
40 Cardiac Airbag Technical Manual Figure 4. Sys tem Status 2.6.6 Holter Me mory ort nt information is available with in the Holter memory. The Holter m emory has a preset configu ration to provide the most criti cal nformation to the physician. - Page 47 Cardiac Airbag Technical Manual 41 Figure 5. Episode List Stored IEGM - The ICD can store up to 30 minutes of two channel intracardiac electrogr ams (IEGMs) including the history and prehistory of the following events: • Detection • Redetection •...
-
Page 48: Arrhythmia Induction Features
42 Cardiac Airbag Technical Manual Figure 6. Stored IEGM 2.6.7 Arrhythmia Induction Features Cardiac Airbag IC D offers two arrhythmia induction methods for non-invasive EP testing. These include the following: HF Burst Induction This feature consists of a large number of pulses delivered in rapid succession over a period of several seconds. -
Page 49: Manual Shock
Cardiac Airbag Technical Manual 43 2.6.8 Manual Shock The Cardiac Airbag ICD can deliver a manual shock on demand through a programmer command in the EP test menu. deliver a shock, place the wand over the device and select the Start Shock button. -
Page 50: Software Features
3. Software Features This section describes the features available with A-K00.0.U programmer software and the procedures necessary for interrogating and programming Cardiac Airbag ICDs. All references to Cardiac Airbag are also applicable to the Cardiac Airbag-T. PLUS PLUS Please refer to the TMS 1000... -
Page 51: Interrogate Icd Without Follow-Up
Cardiac Airbag Technical Manual 45 Figure 7. Follow-Up Assistant with measured values 3.1.1 Interrogate ICD withou t Follow-up To perform an interrogation without using the Follow-Up Assis n ta t, select Skip Follow-Up Interrog ate ICD. Once interrogation is completed all current programmed parameters list d within the Parameter screen. - Page 52 46 Cardiac Airbag Technical Manual [Follow-Up] - The Follow-up Assistant (FAST) is incorporated into the programmer applications to provide a user-defined system guided follow-up. The “guided” follow-up function was developed to allow consistent and quick actions by the user through a single command.
-
Page 53: Parameter Window
Cardiac Airbag Technical Manual 47 [Emergency] - The [Emergency] function key is always present. Activating this key produces a screen display that turns ventricular tachyarrhythmia Detection ON and OFF, activates emergency bradycardia pacing parameters, or activates a programmer commanded shock. - Page 54 48 Cardiac Airbag Technical Manual Figure 8. [Parameter] Window...
- Page 55 Cardiac Airbag Technical Manual 49...
-
Page 56: Sterilization And Storage
50 Cardiac Airbag Technical Manual 4. Sterilization and Storage The ICD is shipped in a storage box, equipped with a quality control seal and product information label. The label contains the model specifications, technical data, serial number, use before date, and sterilization and storage information. - Page 57 Cardiac Airbag Technical Manual 51...
-
Page 58: Implant Procedure
Perform an interrogation of the ICD. Ensure programmer operation, nominal device parameters and battery status is appropriate for a new Cardiac Airbag ICD. Program Detection and Therapy to “Disabled” prior to handling the Cardiac Airbag ICD. Sufficient training on the device and its associated components is required prior to implanting the ICD. -
Page 59: Opening The Sterile Container
Refer to the appropriate lead system technical manual. 5.3 Opening the Sterile C ontai The Cardiac Airbag ICDs are packaged in two plastic containers, one within the other. Each is individually sealed and then sterilized with ethylene oxide. Due to the double packing, the outside of the inner container is... -
Page 60: Lead To Device Connection
54 Cardiac Airbag Technical Manual 5.5 Lead to Device Connection The Cardiac Airbag ICDs have been designed and are recommended for use with a defibrillation lead systems having one IS-1 connector for ventricular sensing and pacing and up to two DF-1 connectors for delivery of shock therapy. -
Page 61: Blind Plug Connection
After carefully retracting the torque wrench, the perforation will self-seal. 5.6 Blind Plug Co nnection he Cardiac Airbag ICDs come with a blind plug (pre inserted) in an unused header port. Refer to the following steps when connecting blind plugs to the device. -
Page 62: Program The Icd
56 Cardiac Airbag Technical Manual 1. Confirm that the setscrews are not protruding into the connector receptacles. To retract a setscrew, insert the enclosed torque wrench through the perforation in the self-sealing plug at an angle perpendicular to the lead connector until it is firmly placed in the setscrew. -
Page 63: Suggested Cardiac Airbag Implant Procedure
4. Run a baseline ECG 5. Ensure that an external defibrillator is connected to the patient. Preparing the ICD 1. Select a Cardiac Airbag ICD package. 2. Place the programming wand over the ICD. A green flashing light indicates... - Page 64 58 Cardiac Airbag Technical Manual Figure 10 . System Status Performing a manual capacitor reformation 1. Press Options button. Select Start Formation (See Figure 11.) and then 3. During charging, an ICD Charging meter is displayed in the ICD Status box.
- Page 65 Cardiac Airbag Technical Manual 59 Figure 11. Manual Cap Reform PLUS essing the TMS 1000 for lead and device-based test 1. Press Options button. 2. In the Programmer area, press Implant List button to return to the main programmer screen.
- Page 66 60 Cardiac Airbag Technical Manual Measuring R-wave Amplitude 1. Select Intracardiac Measurements 2. Press and hold the Record Data (See Figure button to measure the R-wave amplitude. There is an audible tone when the ICD has detected a sensed R-wave.
- Page 67 Cardiac Airbag Technical Manual 61 Determining ventricular capture thresholds 1. Verify VVI mode is highlighted. 2. Set the Lower Rate at 5 to 10 ppm above the patient’s intrinsic rate. Press Start Pacing to begin threshold testing at the displayed parame ters.
- Page 68 HV 1 Shock pin = HV 1 {distal coil} HV 2 Shock pin = HV 2 {proximal coil} 8. Tighten each set-screw using a BIOTRONIK torque wrench until a clicking sound is heard. 9. Insert the ICD into the pocket with etching facing up.
- Page 69 Cardiac Airbag Technical Manual 63 Performing device-based testing measurements 1. Press Implant List button. 2. Place a sterile cover over the programming wand and instruct the implanting physician to position the wand over the device 3. A green flashing light on the wand...
- Page 70 64 Cardiac Airbag Technical Manual DFT testing 1. Select method to induce ventricular fibrillation with the ICD: Shock on T wave or HF burst. Press Print button to be gin printing induction. 3. Press Start VT/VF Induction (See Figure 14) and OK to induce VF.
- Page 71 Cardiac Airbag Technical Manual 65 Figure 15. VF Induction Test 6. Read Shock Data is automatically updated with shock values. 7. Press Print to print out the DFT Test Screen. 8. Select Holter and review EGM to ensure proper sensing during VF.
- Page 72 66 Cardiac Airbag Technical Manual Final Programming 1. Verify final programming following parameters: detection intervals, pacing, sensing, and therapy. 2. Select Transmit to permanently program final parameters. 3. Verify VT/VF Detection is Enabled. 4. Select Parameter button and press Interrogate then Print to print out final programming and system status.
- Page 73 Cardiac Airbag Technical Manual 67...
-
Page 74: Follow-Up Procedures
More → Preferences → PC from the implant screen 4. Connect the patient to the surface ECG cable. 5. Place the programming wand over the ICD. A green flashing light indicates good telemetry communication. 6. The Cardiac Airbag is automatically interrogated with the wand over the ICD. - Page 75 Cardiac Airbag Technical Manual 69 Starting Follow-Up Assistant 1. The software begins by displaying the Follow-Up Assistant screen. (See Figure 16) (To fully activate the Follow-Up Assistant program, ensure that all boxes are checked.) 2. Press Start Follow-Up, located in the lower left corner of the screen.
- Page 76 70 Cardiac Airbag Technical Manual Determining the ventricular pa cing threshold 1. Enter Tests→Threshold (See Figure (automatic through Follow-up Assistant.) Set Rate at 5 to 10 ppm above the patient’s intrinsic rate. 3. If desired, turn Print Test ON to run paper during testing.
- Page 77 Cardiac Airbag Technical Manual 71 Figure 17. Pacing Threshold Test Printing and analyzing detailed Holter data information 1. Press the Holter (See Figure 18) button to view episodes. 2. From the Episode List, select the episode EGM you wish to view and/or print.
- Page 78 72 Cardiac Airbag Technical Manual Figure 18. Holter EGM Episode Verify system status 1. Press Follow-up button. Check R A mplitudes (greater than 5 mV) 3. Verify Pacing Impedance values are within an acceptable range (300-10 00Ω) 4. Verify Battery Status is OK.
- Page 79 Cardiac Airbag Technical Manual 73 Figure 19. System Status 3. Verify remaining shocks to ERI by checking the level of the episode gauge. 4. ERI is reached when 3 treated VF episo des have occurred. 5. At ERI, contact your...
-
Page 80: Longevity
2.4 volts and 500 ohm pacing pedance and all shocks at maximum energy (30 joules) at 37C. The estimates for service time are based on a 24 month elf life, therefore, if the Cardiac Airbag ICD is implanted prior to the... - Page 81 Cardiac Airbag Technical Manual 75 In this table, it is assum ed that the device delivers no shocks to treat ventricular tachyar rhythmias, however, automatic capacitor reformations are equally spaced on a quarterly (every 3 months) basis throughout the life of the ICD. Therefore, the table starts at a minimum of 4 shocks per year, because capacitor reformations are equivalent to shocks.
-
Page 82: Treated Vf Episode Eri Method
76 Cardiac Airbag Technical Manual 6.3.2 Treated VF Episode ERI Method The Cardiac Airbag ICD is designed to treat a limited number (3) of spontaneous VF episodes prior to reaching ERI. Induced VF episodes via the BIOTRONIK programmer that receive shock therapy with the programmer wand in place do not increment against the 3 treated VF episode limit. - Page 83 Cardiac Airbag Technical Manual 77 Figure 21. Status at ERI Upon reaching ERI, the battery has enough energy left to continue monitoring for at least three months and to deliver minimum of six high energy shocks. The estimates associated with duration of ERI assume the ICD is sensing an intrinsic sinus rhythm at a rate of 70 bpm.
-
Page 84: Explantation
6.4 Explantation Explanted ICDs, lead systems, and accessories may not be reused. Please complete the appropriate out of service (OOS) form and return it to BIOTRONIK with the explanted devices. All explanted devices should be sent packaged in a bio-hazard container. - Page 85 Cardiac Airbag Technical Manual 79...
-
Page 86: Technical Specifications
80 Cardiac Airbag Technical Manual 7. Technical Specifications The following are the technical specifications for the Cardiac Airbag ICDs. The ranges are presented in the format: x…(y)…z where x = the lowest value, y = the increment, and z = the largest value. - Page 87 Cardiac Airbag Technical Manual 81 Parameters - Tachyarrhythmias Parameter Value Range Detection Parameters for VT Monitoring Zone Rate (VT Monitoring) OFF; 270…(10)…600 ms 100… 222 bpm Interval Counter: Initial Detection Interval Counter: Redetection Onset 20 % (adaptive) Stability ±24 ms (absolute)
- Page 88 82 Cardiac Airbag Technical Manual Bradycardia Therapy Parameter Value Range Mode VVI, VVIR, OVO (OFF) Rate 30…(5)…120 ppm Amplitude 0.2…(0.1)…6.2, 7.5 V Pulse Width 0.5, 1.0, 1.5 ms Maximum Sensor Rate 100, 125 ppm Sensor Gain Sensor Threshold mean Rate Increase...
- Page 89 Cardiac Airbag Technical Manual 83 Federal Communications Commission Disclosure The Belos -T ICD is eq uipped with an RF transmitter for wireless communications. T his transmitter is authorized by rule un der the Medica Implant Communications S ervice (47 CFR Part...
- Page 90 84 Cardiac Airbag Technical Manual...
-
Page 91: Appendix A
Cardiac Airbag Technical Manual 85 Appendix A Connector Compatibility Cardiac Airbag ICDs are indicated for use only with commercially available BIOTRONIK bipolar ICD lead systems or other lead systems with which it has been tested. The Cardiac Airbag family of ICDs is mechanically compatible with: •... - Page 92 Distributed by: BIOTRONIK, Inc. 6024 Jean Road Lake Oswego, OR 97035-5369 (800) 547-0394 (24-hour) (503) 635-9936 (FAX) Manufactured by: BIOTRONIK GmbH & Co. Woermannkehre 1 D-12359 Berlin Germany M4088-A 3/03...














Need help?
Do you have a question about the Cardiac Airbag and is the answer not in the manual?
Questions and answers