Advertisement
Quick Links
Edwards SAPIEN XT
Transcatheter Heart Valve with the NovaFlex+ Delivery System
Instructions for Use – Pulmonic
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician.
Implantation of the Transcatheter Heart Valve (THV) should be performed only by physicians who
have received Edwards Lifesciences training. The implanting physician should be experienced in
balloon valvuloplasty.
Please verify that you have the latest version of the instructions for use prior to using the device
by visiting http://THVIFU.edwards.com or by calling 1.800.822.9837. In order to access the
instructions for use, an IFU Code will be required.
STERILE: The THV is supplied sterilized with glutaraldehyde solution. The delivery system is
supplied sterilized with ethylene oxide gas.
Edwards Lifesciences, the stylized E logo, Edwards, Carpentier-Edwards, Edwards SAPIEN, SAPIEN,
Edwards SAPIEN XT, SAPIEN XT, NovaFlex, NovaFlex+, Qualcrimp, and ThermaFix are trademarks of
Edwards Lifesciences Corporation.
All other trademarks are the property of their respective owners.
1
Advertisement

Summary of Contents for Edwards SAPIEN XT
- Page 1 Edwards Lifesciences, the stylized E logo, Edwards, Carpentier-Edwards, Edwards SAPIEN, SAPIEN, Edwards SAPIEN XT, SAPIEN XT, NovaFlex, NovaFlex+, Qualcrimp, and ThermaFix are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
-
Page 2: Device Description
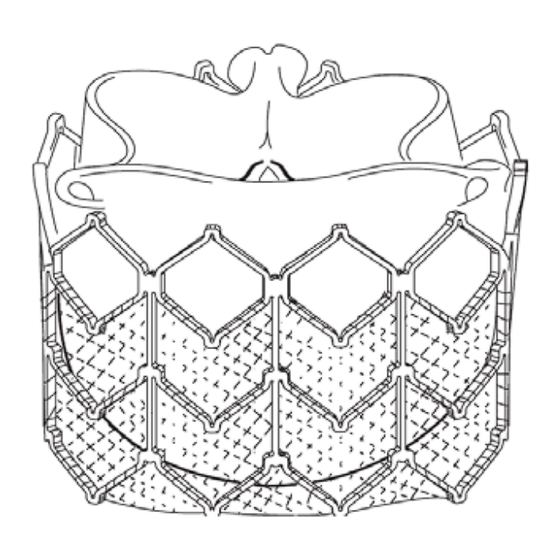
NovaFlex+ Delivery System (Figures 2a, 2b, 2c) The NovaFlex+ delivery system (usable length 105 cm) is used for delivery of the Edwards SAPIEN XT THV. The delivery system includes a flex wheel for articulation of the flex catheter, a tapered tip at the distal end of the delivery system to facilitate advancing to the RVOT, and a balloon catheter for deployment of the THV. - Page 3 Figure 3 Laminated Qualcrimp Indications The Edwards SAPIEN XT Transcatheter Heart Valve (THV) Systems are indicated for use in pediatric and adult patients with a dysfunctional, non-compliant Right Ventricular Outflow Tract (RVOT) conduit with a clinical indication for intervention and: •...
-
Page 4: Potential Adverse Events
For more information about glutaraldehyde exposure, refer to the Material Safety Data Sheet available from Edwards Lifesciences. • Patient anatomy should be evaluated to prevent the risk of access that would preclude the delivery and deployment of the device. - Page 5 • Renal insufficiency or renal failure • Conduction system defect Arrhythmia • Arteriovenous fistula • Reoperation or reintervention • Ischemia or nerve injury • Pulmonary edema • Pleural effusion • Bleeding • Anemia • Abnormal lab values (including electrolyte imbalance) •...
-
Page 6: Directions For Use
(or equivalent) Inflation devices provided by Edwards Lifesciences Edwards Crimper 9350CR Includes the Qualcrimp Crimping Accessory and 2-piece Crimp Stopper ** Or other compatible sheath provided by Edwards Lifesciences Additional Equipment: • 20 cc syringe or larger (x2) • 50 cc syringe or larger •... - Page 7 50 cc or larger syringe. Slowly release the plunger and leave zero-pressure in the system. Close the stopcock to the delivery system. By rotating the knob of the inflation device provided by Edwards Lifesciences, transfer the contrast medium into the syringe to achieve the appropriate volume required to...
- Page 8 Contrast media usage should be monitored. CAUTION: Procedure may require venous cut-down with surgical closure of the puncture site due to the size of the venotomy. 7.3.1 Valvuloplasty Refer to Edwards Balloon Catheter Instructions for Use (IFU) for information on device preparation and handling.
- Page 9 7.3.2 THV Delivery Step Procedure Dilate the access site using the Edwards Dilator Kit, if needed. Refer to the Edwards Dilator Kit IFU for information on device preparation and handling. Ensure the guidewire placement is via the left pulmonary artery, unless precluded by patient anatomy.
-
Page 10: How Supplied
Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2 W/kg (Normal Operating Mode). Under the scan conditions defined above, the SAPIEN XT THV is expected to produce a maximum temperature rise of 2.6 °C after 15 minutes of continuous scanning. -
Page 11: Clinical Studies
THV, in the COMPASSION trial. However, there is extensive clinical evidence on Edwards SAPIEN and SAPIEN XT THVs in the aortic position from the PARTNER I and II trials. Information on the PARTNER I and II trials may be found on the FDA website: SAPIEN: http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100041b.pdf... - Page 12 COMPASSION Clinical Data The demographics and baseline characteristics of the safety population are summarized in Clinical Table Clinical Table 2: Demographics and Baseline Characteristics (Safety Population, N=79) Characteristic Statistic Age - yr 28.0 ± 13.97 (79) 25.0 (10.0, 72.0) <12 Years(Child) 3 / 79 (3.8%) 12-21 Years (Adolescent) 26 / 79 (32.9%)
- Page 13 COMPASSION Results The primary endpoint was freedom from device- or procedure-related death and/or reintervention at 1 year for the valve implant population, which was 97.1% (Clinical Table 3) and met the prespecified performance goal of 75%. There were no device- or procedure-related patient deaths at one year, and the incidence of reinterventions solely contributed to the KM estimate.
- Page 14 Clinical Figure 2 presents the freedom from reintervention to 5 years for the valve implant population, stratified by type of reintervention: a) freedom from surgical pulmonic valve repair was 98.3% at 1 year and 91.8% at 5 years; b) freedom from transcatheter pulmonic valve implantation was 97.1% at 1 year and 85.8% at 5 years;...
- Page 15 The secondary endpoints were defined as freedom from Major Adverse Cardiac and Cerebrovascular Events (MACCE) at 6 months, and functional improvement at 6 months. Freedom from MACCE at 6 months in the valve implant population was 94.1% (see Clinical Table 4). Clinical Table 4: Freedom from MACCE at 6 Months (Valve Implant Population) Patients Standard...
- Page 16 The site-reported serious adverse events are shown in Clinical Table 6. Clinical Table 6: Incidence of Site-Reported Serious Adverse Events by Study Visit (with CEC adjudication where available) (Safety Population, (N=79)) <= 30 Days 31 – 365 Days All Events Patients with Patients with Patients with...
- Page 17 The key CEC-adjudicated adverse events to 5 years are presented in Clinical Table 7. Clinical Table 7: CEC-Adjudicated Endpoint Adverse Events (Valve Implant Population, N=69) 5 Years 30 Days 1 Year 2 Year 3 Years 4 Years (Patients at (Patients at (Patients at (Patients at (Patients at...
- Page 18 Valve Frame Fractures No patient had a study valve frame fracture. Valve Dysfunction Valve dysfunction is a non-hierarchical composite of all-cause mortality, surgical pulmonary valve replacement, valve frame fracture, recurrent pulmonary stenosis, moderate/severe regurgitation and reintervention. Clinical Figure 4 presents the freedom from valve dysfunction, which was 94.2% at 1 year and 65.7% at 5 years.
- Page 19 All Cause Mortality Clinical Figure 5 presents the freedom from all-cause mortality for the valve implant population, which was 100% at 1 year and 97.8% at 5 years. Clinical Figure 5: Freedom from All-Cause Mortality to 5 Years (Valve Implant Population)
- Page 20 Endocarditis Clinical Figure 6 presents the freedom from endocarditis for the safety population, which was 97.3% at 1 year and 86.1% at 5 years. Patients that were pre-stented had an observed rate of endocarditis of 4.2% (3/71) whereas patients that were not pre-stented had no reported endocarditis (0/7). Due to the small number of patients that were not pre-stented, no statistical conclusions may be made from this data.
- Page 21 Subgroup Analyses By Pre-stenting Pre-stenting of the RVOT conduit for the SAPIEN THV was performed during the IDE study, at the discretion of the treating physician. The protocol did not specify any criteria for how or when to perform the pre-stenting procedure. Therefore, the data presented below represents the outcomes of subjects who were implanted with a variety (in number and type) of stents along with the SAPIEN THV.
- Page 22 Clinical Table 8: Incidence of Site-Reported Serious Adverse Events by Study Visit By Stenting (Safety Population) No Stent (N=7) Stented (N=71) <= 30 Days 31 – 365 Days All Events <= 30 Days 31 – 365 Days All Events Patients with Patients with Patients with Patients with...
- Page 23 By Patient Age Clinical Table 9 shows the site-reported serious adverse events grouped by age at baseline (≤ 21 or ≥ 22 years of age). Note that this study was not designed to investigate the differences in outcomes between age groups. Clinical Table 9: Incidence of Site-Reported Serious Adverse Events by Study Visit by Baseline Age Group (Safety Population) Age 21 or Younger (N=29)
- Page 24 Clinical Table 9: Incidence of Site-Reported Serious Adverse Events by Study Visit by Baseline Age Group (Safety Population) Age 21 or Younger (N=29) Age 22 or Older (N=50) <= 30 Days 31 – 365 Days All Events <= 30 Days 31 –...
- Page 25 Clinical Table 10 presents a summary of freedom from CEC-adjudicated MACCE by baseline age group for the VI population. Clinical Table 10: Summary of Freedom from CEC-Adjudicated MACCE by Baseline Age Group (VI Population) Kaplan-Meier Survival Estimate [1] 30 Days 6 Months 1 Year 2 Year...
- Page 26 Clinical Table 11 presents overall functional improvement at 6 months by baseline age group in the VI population. Clinical Table 11: Functional Improvement at 6 Months by Baseline Age Group (VI Population) Functional Improvement Age 21 or Younger (N=27) Age 22 or Older (N=42) Overall Functional Improvement [1] 17 / 22 (77.3%) 34 / 36 (94.4%)
- Page 27 presents the overall functional improvement through 5 years of follow-up by age group for the VI population. Clinical Table 14 Clinical Table 14: Functional Improvement at Follow-Up by Age Group (VI Population) Group Functional Improvement Days Months Year Years Years Years Years Age 21 or...
- Page 28 By Valve Size Clinical table 10 shows the site-reported serious adverse events stratified by valve size. Note that this study was not designed to investigate the differences in outcomes between valve sizes. Also note that the 29 mm valve size was not evaluated in this study. Clinical Table 15: Incidence of Site-Reported Serious Adverse Events by Study Visit By Valve Size (Safety Population) 23 mm Valve (N=48)
- Page 29 Clinical Table 15: Incidence of Site-Reported Serious Adverse Events by Study Visit By Valve Size (Safety Population) 23 mm Valve (N=48) 26 mm Valve (N=22) <= 30 Days 31 – 365 Days All Events <= 30 Days 31 – 365 Days All Events Patients with Patients with...
- Page 30 02/2016 Copyright 2016, Edwards Lifesciences LLC All rights reserved. Manufacturer Web IFU Edwards Lifesciences LLC Telephone 949.250.2500 10001249001 A One Edwards Way 800.424.3278 Irvine, CA 92614-5688 USA 949.250.2525 Made in USA...











Need help?
Do you have a question about the SAPIEN XT and is the answer not in the manual?
Questions and answers