Össur COLD RUSH COMPACT Manual
- Instructions for use manual (14 pages)
Advertisement

SYMBOLS
 | Caution Symbol Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences |
 | BF Applied Part |
 | Manufacturer address |
 | Consult instructions for use |
 | Protected against solid foreign objects of 12.5mmø and greater. Protected against vertically falling water drops. |
 | Negative center connection (ISO. 5926) |
 | Preferred Output Polarity Symbol |
 | Class II Equipment |
 | For indoor use only |
DESCRIPTION
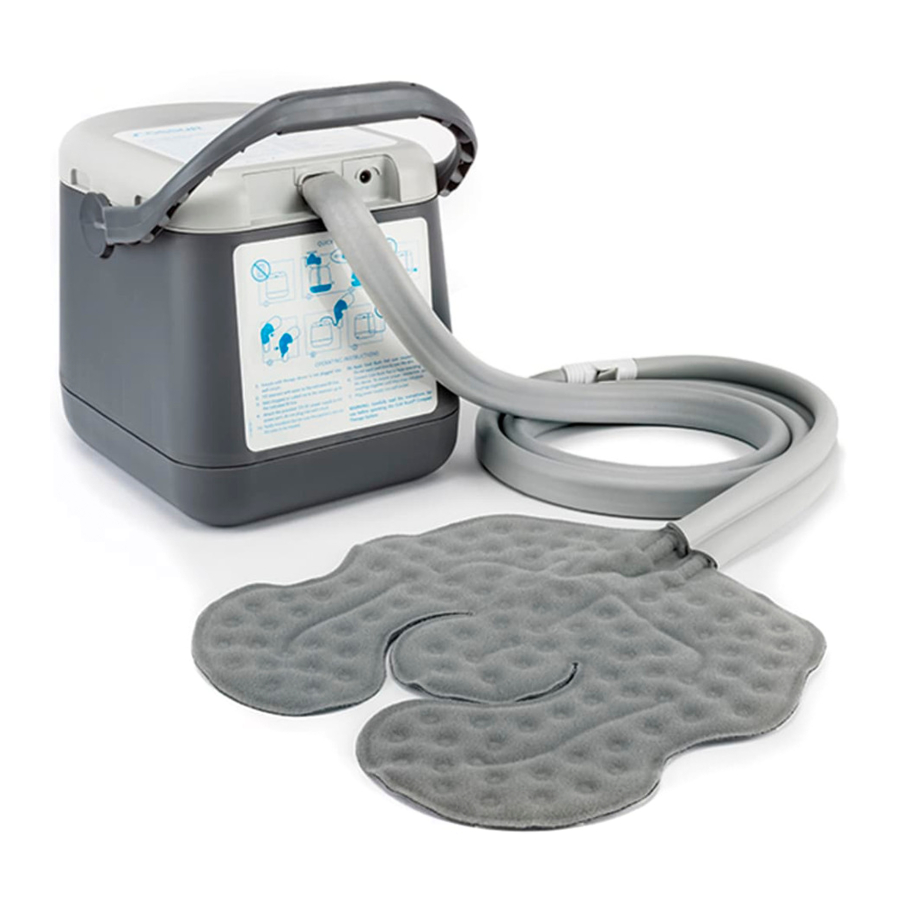
Cold Rush Compact is a mechanical circulation cold therapy device. The device is composed of a bucket holding a mixture of water and ice, a circulation pump that forces the coolant through a closed-loop hydraulic circuit and a cooling pad that is used to apply cold therapy to the region that is being treated. The system operates on a 12-volt power which is supplied via an external wall-mounted power supply.
LIST OF PARTS
Fig. 1:

- Cold Rush Compact Cold Therapy System
- Hose
- Couplings
- DC Connector
- 12V Power Supply
- Cold Rush Pad (Sold Separately)
INTENDED PURPOSE
The device is non-invasive, intended for single patient use and designed to reduce localized pain and swelling following surgical procedures or trauma.
Indications for use
- Provides cold therapy
- Indications requiring the application of cold therapy to a joint or limb such as post-surgical, trauma pain, and swelling
Contraindications
The device should not be used on patients with the following conditions:
- Known hematological dyscrasias that predispose to thrombosis (e. g., paroxysmal cold hemoglobinuria, cryoglobulinemia, sickle cell disease, serum cold agglutinins)
- Compromised local circulation (including arteriosclerosis, ischemia)
- Neurologic impairment (including paralysis or localized compromise due to multiple surgical procedures or diabetes) in the affected region, polyneuropathy, or other nerve damage causing decreased skin sensitivity
- Cognition or communication impairments that prevent the user/ patient from giving accurate and timely feedback including incapacitated patients with severe cardiovascular disease, anesthetic skin, hypercoagulation disorders, poor circulation, extremities sensitive to pain, extremely low blood pressure
- Raynaud's disease, phenomenon, or other vasospastic conditions
- Buerger's disease, significant vascular impairment
- Hypersensitivity to cold (cold urticaria) or history of cold related injury (including chilblains or frostbite)
- Severe cardiovascular disease, anesthetic skin, hypercoagulation disorders, poor circulation, extremities sensitive to pain, extremely low blood pressure, incapacitation, decreased skin sensitivity, vein ligation or recent skin grafts, or pheochromocytoma
- Localized unstable skin condition (e. g., dermatitis, vein ligation, gangrene, or recent skin graft) in the affected region or potential impairment of healing in the treatment area, including infection
- Tissues inflamed as a result of recent injury or exacerbation of chronic inflammatory condition
Warnings and Cautions:
This product has been designed and tested based on single patient usage and is not recommended for multiple patient use. If any problems occur with the use of this product, immediately contact your healthcare professional.
The device can be cold enough to seriously injure skin. Read the instructions which are located inside the unit, the instructions for the pad, and the labels on the device carefully before operating the device.
RX ONLY
This device should only be used with a prescription from a physician that includes the following treatment information:
- the number of days or weeks that the treatment should last and
- the length and frequency of product use (and breaks) during treatment,
- instructions about how to inspect the skin, and
- frequency of skin checks.
A A blank treatment protocol to be filled out by your healthcare provider is provided on top of the device.DO NOT USE THIS DEVICE IF YOU DO NOT HAVE A PRESCRIPTION FROM YOUR PHYSICIAN THAT INCLUDES ALL SUCH TREATMENT INFORMATION
POSSIBLE COLD-RELATED INJURIES
This device reduces the temperature of the skin and tissue. If not used properly and in accordance with the prescribed instructions from your physician, this device can cause serious injury, non-freezing cold injury, tissue necrosis and nerve damage.
SKIN INSPECTION AND ADVERSE REACTIONS
You or your healthcare provider must check your skin at least every 1–2 hours during use of this device, regardless of whether you are asleep or awake, for any change in skin condition, including for any increase in pain, burning, blistering, itching, increased swelling, discoloration of the skin, increased redness, welts or any other changes in the appearance of the skin. If you experience any of these reactions, you should immediately discontinue use of your device and contact your healthcare provider. Any dressing, casting, bracing or wrapping must not interfere with your ability to check your skin condition. If you are unable to check your skin condition for any reason, do not use the device.
NO DIRECT CONTACT WITH SKIN: INSULATION BARRIER REQUIRED (not included).
The Cold Rush Pad should never make direct contact with the skin. The pad operating temperature is too cold to be placed directly on the skin. An insulation barrier between the pad and the skin should be used at all times without exception so as to prevent the skin from becoming too cold. Failure to use an insulation barrier between the pad and the skin can lead to a cold-related injury.
- Unit is not suitable for use in the presence of flammable anesthetic mixture with air, oxygen, or nitrous oxide.
- Follow local ordinances or regulations for proper disposal of device, accessories, and packaging
Federal law requires this device to be sold by a physician or by the order of a physician.
USE ONLY ÖSSUR COLD RUSH PADS
Competitive brand cold therapy pads should not be used with the device as the temperature may become too cold when using competitive pad and the pads may not otherwise function properly, causing injury. Only Össur Cold Rush Pads should be used with the device.
NO MOISTURE IN INSULATION BARRIER
If the moisture is present in the insulation barrier, the skin may become colder than intended. The insulation barrier should be inspected regularly to check for moisture caused by bleeding, sweating or condensation. If moisture is found on the insulation barrier between the pad and the skin, immediately discontinue use of the device until the moisture is removed and a fresh barrier is placed between the pad and the skin.
Risk factors
Physicians should carefully consider the following conditions or factors before prescribing the device:
- Sensitivity to cold;
- General impaired circulation;
- Use of medications that may negatively affect peripheral vascular circulation, including beta adrenergic blockers and local epinephrine use (such as local anesthetics);
- Poor nutrition, smoking, tobacco use, excessive use of caffeine or alcohol and any other behavior negatively affecting circulation;
- Desensitization of the treatment area due to local anesthesia or regional nerve blockers;
- Cognitive impairment and use of medications that have a negative effect upon mental capacity or judgment;
- Moisture at the application site due to excessive bleeding, sweating or condensation;
- Diabetes;
- Use of product on hand, wrist, foot or ankle; and
- Young children and elderly.
Water level should not exceed the reference mark on the sticker inside the bucket to avoid risk of overflow when closing the cover.
Make sure the ice level does not exceed the reference mark on the sticker. Failure to comply will cause device overflow when closing the lid.
Healthcare provider responsibilities

- The prescribing physician must determine (1) the number of days or weeks that the treatment should last and (2) the length and frequency of product use (and breaks) during treatment. All such information should be included in the patient's prescription.
- Appropriate training must be provided by the healthcare provider, in the proper application, use and operation and care of the device.
- The healthcare provider must monitor the patient's use of the device to assure compliance with the prescribed protocol, appropriate use, proper application, and operation of the device, including but not limited to application and maintenance of an insulation barrier between the patient's skin and the pad.
Additional warnings
- The device should never be left unattended when plugged in.
- Do not place the tubes or the power cord where your or other's feet can get entangled, causing a fall.
- Never drop or insert any object into any opening or hose.
- Do not operate where aerosol (spray) products are being used or where oxygen is being administered.
- Do not attempt to drag or reposition the device by pulling or tugging on the insulated hoses, as this may cause damage to the device and/or cause the hoses to disconnect. Use handle when moving the device.
- Do not place the tubes or the power cord by the top of your bed where they could twist around your neck while you are sleeping.
- The pump in the device is designed to run with water. Running the device without water will cause permanent damage to the pump.
- A wireless communications equipment such as wireless home network devices, mobile phones, cordless telephones and their base stations, walkie-talkies can affect the device and should be kept at least a distance 3.3 m away from the device.
Electric shock hazard
To create the safest operating conditions possible, Össur has designed the device to operate on a 12-volt power supply, significantly reducing the chance of dangerous electric shock. Avoid use near any water source.
- Only use the power supply provided with the device.
- Unplug device before filling it with ice and water.
- To avoid danger of electric shock be certain that hands are dry before inserting or removing the power supply from wall socket.
- Avoid getting water or ice on the power adapter.
- Always unplug this product immediately after each use.
- Never use the device while bathing.
- Do not place or store product where it can fall or be pulled into a tub or sink.
- Do not place in or drop into water or other liquid.
- Do not reach for a product that has fallen into water. Unplug immediately.
- Keep cord & device away from heated surfaces.
- Never operate this product if:
- The device has a damaged electrical cord or plug;
- The device has been dropped or damaged;
- The has been dropped into water; or
- The device is not working properly.
- If any of the above have occurred, immediately discontinue use of the device and return the product to your healthcare provider for examination and repair.
USAGE
Setup and operation
- Do NOT plug unit into wall socket until steps 2–7 are completed.
- Fill reservoir with water to the indicated fill line (Fig. 2).
water level should not exceed the reference mark on the sticker inside the bucket to avoid risk of overflow when closing the cover.
![Össur - COLD RUSH COMPACT - Setup and operation - Step 1 Setup and operation - Step 1]()
- Add chopped or cubed ice to the reservoir up to the indicated fill line (Fig. 3).
Make sure the ice level does not exceed the reference mark on the sticker. Failure to comply will cause device overflow when closing the lid.
![Össur - COLD RUSH COMPACT - Setup and operation - Step 3 Setup and operation - Step 3]()
- Close the reservoir. With handle in the open position, press lid firmly onto unit making certain that the lid is in full contact with the vessel and the seal is engaged, then pull handle into the locked position (Fig. 4)
![Össur - COLD RUSH COMPACT - Setup and operation - Step 4 Setup and operation - Step 4]()
- Attach the provided 12V power supply to DC power port, but do not plug into wall socket (Fig. 5).
![Össur - COLD RUSH COMPACT - Setup and operation - Step 5 Setup and operation - Step 5]()
- Apply insulation barrier (not included) over the patient's skin in the area to be treated (Fig. 6).
![Össur - COLD RUSH COMPACT - Setup and operation - Step 6 Setup and operation - Step 6]()
- Connect Cold Rush Pad to hose extending from the device. To ensure proper connection, push couplings together until they snap into place (Fig. 7).
![Össur - COLD RUSH COMPACT - Setup and operation - Step 7 Setup and operation - Step 7]()
- Apply pad over insulation barrier (not included). The pad should never be applied directly over the patient's skin (Fig. 8).
![Össur - COLD RUSH COMPACT - Setup and operation - Step 8 Setup and operation - Step 8]()
- Plug the provided 12V power supply to wall socket (120V US, 240V Europe) (Fig. 9).
![Össur - COLD RUSH COMPACT - Setup and operation - Step 9 Setup and operation - Step 9]()
- To turn off the cold therapy device, unplug the power supply unit from wall socket.
- Always turn the cold therapy device off before disconnecting the pad or any hoses. To disconnect the hoses, press the release tabs on the coupling and pull the connectors apart.
- To open lid, press handle down to disengage lid seal, then lift to remove(Fig. 10)
![Össur - COLD RUSH COMPACT - Setup and operation - Step 10 Setup and operation - Step 10]()
- Drain all of the water out of the cold therapy device after each use.
- Follow directions 2–8 before each use.
Cleaning and prolonged storage
When you have finished all of your cold therapy treatments, prior to prolonged storage, fill the device so the water level is at the ice fill line.
- Add 2 tablespoons of liquid bleach or 3 ounces of 3% hydrogen peroxide to the water and mix.
- Connect hoses to the Cold Rush Pad, plug in the device to start.
- Allow water mixture to circulate for 5 minutes.
- To turn device off, unplug power supply.
- Carefully drain the water mixture out of the device and store in a dry, cool, and dark place leaving the lid slightly open. (Exposure to sunlight and extreme heat may damage hoses and device.)
- When reusing the device after storage, fill the reservoir with clean water and turn the pump on to circulate the water through the hoses and flush the remaining water mixture out of the system (use old pad for this procedure, discard the pad after cleaning is completed).
- Dispose water and refill the reservoir with fresh ice and water combination to begin cold therapy treatment. Follow operating instructions 2–9 before each use.
- Before you use the device, inspect hoses and new pad for any tears or breaks. Contact your healthcare provider to purchase new Cold Rush Pad.
- Clean or replace insulation barrier as recommended by your healthcare practitioner.
Environmental Conditions
- Operating Temperature: +10°C (+50°F) to 40°C (104°F)
- Operating Humidity: 30% - 75% Relative Humidity
- Operating Atmospheric Pressure: 700–1060 h Pa
- Shipping and Storage Temperature: -20°C (-4°F) to 60°C (140°F)
- Shipping and Storage Humidity: 10% - 90% Relative Humidity, non- condensing
- Shipping and Storage Atmospheric Pressure: 700–1060 h Pa
MAINTENANCE
Do not attempt to repair your device as this may create a user hazard and put the user at risk. Any attempt of repairing the device will automatically void warranty.
Power supply specifications
Do not use any other power supply other than the one provided with the unit.
- Model no: UES06WU-120050SPA
- Input: 120V US, 240V EU
- Input frequency: 60Hz US, 50Hz EU
- Output power: 6W
- Output Voltage: 12V
- Output Current: 0.5A
Documents / ResourcesDownload manual
Here you can download full pdf version of manual, it may contain additional safety instructions, warranty information, FCC rules, etc.
Advertisement






























Need help?
Do you have a question about the COLD RUSH COMPACT and is the answer not in the manual?
Questions and answers