Advertisement
Quick Links
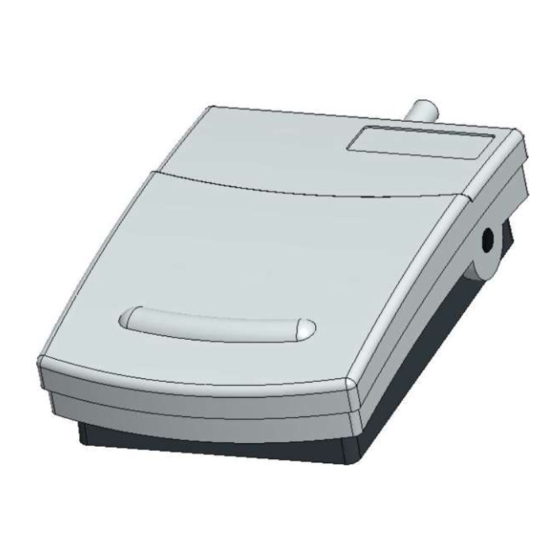
Product name: MKF 2PW-MED
Part-no.:
Specification
neutral pedal in RAL 7035 Light grey
two change-over contacts
3 m shielded cable
Technical Data
Applied standards
Existing certifications
Pedal
Pedal enclosure
Degree of protection
Contact element and material
Connection type
Mechanical lifetime
Dimensions (W x L x H)
Weight
Created on: 05.10.2022
Page
steute Technologies
1 / 3
GmbH & Co. KG
1442721
IEC 60601-1 (Basic Safety)
IEC 60529 (Degrees of protection)
Note: see CSA certificate for corresponding revision level.
CSA
shockproof thermoplastic, self-extinguishing / RAL 7035 Light grey
shockproof thermoplastic, self-extinguishing / black
IPX8 (1m / 35 Min.) according to DIN EN 60529
2 micro switches / gold-plated
3 m (6xAWG 24 UL/CSA RAL 7035)
The expected operating life of the foot switch is limited to 10 years or 1,000,000 switching cycles of the
actuating units, whichever comes first.
73 x 103 x 29 mm
0,3 kg
Brückenstraße 91
32584 Löhne, Germany
Revision of data sheet: -
Phone +49 (0) 5731 745-0
Fax +49 (0) 5731 745-200
D041837
info @ steute.com
www.steute.com
Advertisement

Summary of Contents for steute MKF 2PW-MED
- Page 1 Product name: MKF 2PW-MED Part-no.: 1442721 Specification neutral pedal in RAL 7035 Light grey two change-over contacts 3 m shielded cable Technical Data IEC 60601-1 (Basic Safety) IEC 60529 (Degrees of protection) Applied standards Note: see CSA certificate for corresponding revision level.
- Page 2 Product name: MKF 2PW-MED Part-no.: 1442721 Electrical Ratings max. 25 V AC / 60 V DC Switching voltage (U max. 1 A Switching current (I max. 20 W Switching capacity (P Environmental Conditions Storage/Transport Operating -40 °C up to +70 °C -10 °C up to +60 °C...
- Page 3 Product name: MKF 2PW-MED Part-no.: 1442721 General notes The foot switch as a component of a medical device can be evaluated only together in the overall system of the customer. Therefore the conformity evaluation including the classification of the overall system according to the Medial Device Directive 93/42/EEC respectively EU Medical Device Regulation 2017/745 must be carried out at the customer.





Need help?
Do you have a question about the MKF 2PW-MED and is the answer not in the manual?
Questions and answers