Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Moller Medical Vibrasat power
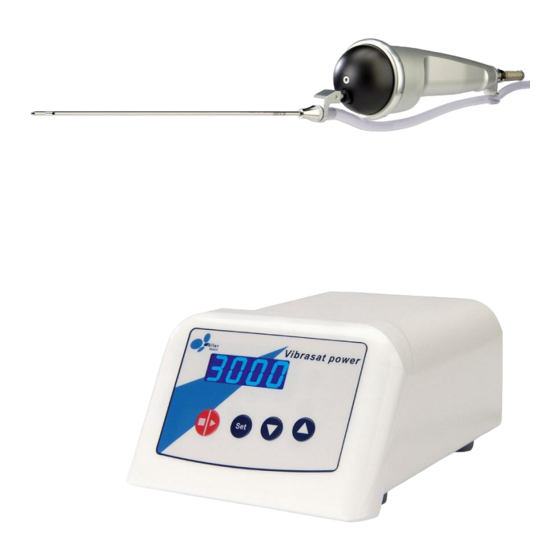
- Page 1 ® Vibrasat power...
- Page 2 IMPORTANT READ CAREFULLY BEFORE USE KEEP THESE INSTRUCTIONS FOR FUTURE CONSULTATION...
-
Page 3: Manufacturer Information
® Vibrasat power Manufacturer information Manufacturer information Möller Medical GmbH Wasserkuppenstraße 29-31 36043 Fulda, Germany Tel: +49 (0) 661 94195 - 0 Fax: +49 (0) 661 94195 – 850 E-mail: info@moeller-medical.com Internet: www.moeller-medical.com © Möller Medical GmbH. All rights reserved. No part of this document may be reproduced or translated in any form or by any means without the prior written permission of Möller Medical GmbH. -
Page 4: Table Of Contents
® Vibrasat power Table of Contents Table of Contents Manufacturer information ................. 3 Table of Contents ....................4 General safety instructions ................6 Explanation of safety symbols used ..............6 Manufacturer's responsibility................9 Precautionary duties of the operator ..............9 Non-Product Additional Equipment .............. - Page 5 ® Vibrasat power Table of Contents Description of the operating elements............21 Handle reprocessing ..................22 Troublesshooting ..................23 Service ......................24 Recurring safety inspections ..............25 10 Disposal ......................26 11 Appendix ....................... 27 Technical Data ....................27 ® Accessories of the Vibrasat power ...............
-
Page 6: General Safety Instructions
® Vibrasat power General safety instructions 1 General safety instructions Explanation of safety symbols used Important information is visually highlighted in the instructions for use. These references are prerequisites for preventing hazards to patients and operating personnel, as well as for avoiding damage to or malfunctioning of the device Symbols used in the instruction manual Caution... - Page 7 ® Vibrasat power General safety instructions “ON” (power) “OFF” (power) Return and disposal according to WEEE Directive Start-stop key SET button for service functions Down button to reduce the number of strokes Up button to increase the number of strokes Attention: Under US Federal law, this device may be only sold to a physician or ordered by a physician.
- Page 8 ® Vibrasat power General safety instructions Manufacture Catalog number Serial number Return and disposal according to WEEE Directive Attention Temperature limit Humidity, limitation Keep dry Stacking limit, do not store more than 4 packs high Use by YYYY-MM-DD Batch code Sterilized using ethylene oxide page 8 of 38...
-
Page 9: Manufacturer's Responsibility
® Vibrasat power General safety instructions Do not re-use Do not resterilise Do not use if package is damaged Contains or presence of phthalates Attention: Under US Federal law, this device may be only sold to a physician or ordered by a physician. Further information on the symbols used can be found on our homepage: www.moeller-medical.com/glossary-symbols Manufacturer's responsibility... - Page 10 ® Vibrasat power General safety instructions tails specified on the nameplate. No liquid must be allowed to penetrate live electrical components. Disconnect the device plug before cleaning. ® Only plug in or unplug Vibrasat power connectors (power cable, foot switch, hand grip) if the device is switched off.
-
Page 11: Non-Product Additional Equipment
® Vibrasat power General safety instructions Non-Product Additional Equipment Any additional equipment that is connected to the analogue and digital interfaces of the device must be proven to meet the requirements of the relevant EN specifications, (e.g. EN60950 for data processing devices, and EN60601 for electrical medical devices). All device configurations must also satisfy the applicable version of the electrical system safety standard EN60601-1. -
Page 12: Warning Section
® Vibrasat power General safety instructions Warning Section This device will not, in and of itself, produce significant weight reduction. This device should be used with extreme caution in patients with chronic medical condi- tions, such as diabetes, heart or lung disease, circulatory diseases or obesity. The volume of blood loss and endogenous body fluid may adversely affect intra and/or postoperative hemodynamic stability and patient safety. -
Page 13: Intended Use (Purpose)
® Vibrasat power Intended use (purpose) 2 Intended use (purpose) ® The Vibrasat power, which consists of a control unit and a handle with connection cable and induces cannulas in vibration, has the purpose of aesthetic body contouring and is used in particular to support the hand movement of the user. -
Page 14: Product Description
® Vibrasat power Product description 3 Product description Each use of the device requires precise knowledge of and compliance with this instruction for use. This instruction for use is not intended to replace instruction of the user by the medical device consultant. The device must be used only by persons who have the re- quired training or knowledge and experience. -
Page 15: Application Description Of The Vibrasat Power
® Vibrasat power Product description ® Application description of the Vibrasat power The device is switched on using the On/Off switch on the device's reverse side via the primary voltage. After the display test, the last set stroke rate value (strokes per minute) appears in the display. -
Page 16: Three-Pedal Foot Switch
® Vibrasat power Product description Three-pedal foot switch The 3-pedal foot switch connected to the back of the device can be used to perform the same stroke rate adjustment functions. The foot switch has the same functions as the "Up", "Down" and "Start/Stop" buttons. On / Off Down –... -
Page 17: Installation And Commissioning
® Vibrasat power Installation and commissioning 4 Installation and commissioning Transport and storage information In order to avoid damage to device and other materials when transporting the device, it is imperative to observe the following safety information. It is imperative to observe transport information on the packaging. ... -
Page 18: Unpacking The Device And Checking The Deliver
® Vibrasat power Installation and commissioning Unpacking the device and checking the deliver ® The Vibrasat power delivery always consists of one carton Ensure that the packaging is delivered undamaged. Transportation damage should be reported immediately to the carrier. ® When unpacking Vibrasat power, ensure that no parts remain in the package. -
Page 19: Assembly
® Vibrasat power Installation and commissioning Assembly Prior operation ensure that the device is cleaned and sterilsed in accordance with the cleaning instructions, see chapter “Handle Preprocessing” page 22. The control unit has to be cleaned in accordance to the hygenic standards. Mild detergents / disinfection fluids (e.g. -
Page 20: Device Connections
® Vibrasat power Installation and commissioning Device connections Connecting socket for hand grip Mains connection with On/Off switch Connecting socket for foot switch Please always observe: Only apply forces to the hand grip in its axial movement direction. Radial force will cause the device to switch off for safety reasons. -
Page 21: Operation
® Vibrasat power Operation 5 Operation ® Description of user for Vibrasat power Any use of the device requires precise knowledge of and compliance with the instructions for use. This instruction for use is not a replacement for user instruction. Only qualified personnel may operate this device. -
Page 22: Handle Reprocessing
® Vibrasat power Handle reprocessing 6 Handle reprocessing See separate instructions: ® Reprocessing of the handgrip Vibrasat power REF 93006873 page 22 of 38... -
Page 23: Troublesshooting
® Vibrasat power Troublesshooting 7 Troublesshooting ® Vibrasat power may not be opened by the operator! ® This chapter identifies some possible problems in connection with Vibrasat power. Several possible solutions are listed for each problem. The first proposed solution is gen- erally the most suitable. -
Page 24: Service
® Vibrasat power Service 8 Service ® Prior to disposal or return of the Vibrasat power, it must undergo a suitable disinfection procedure to exclude possible risk of infection. Consumable materials should be disposed of in accordance with hygiene guidelines. Service information: Never open the device when it is connected to the mains power supply. -
Page 25: Recurring Safety Inspections
® Vibrasat power Recurring safety inspections 9 Recurring safety inspections ® Vibrasat power must be subjected to safety inspections at least every 12 months. Service must immediately repair the device if it is inoperable or unreliable. The safety inspections can be performed by the Service department of Möller Medical GmbH (service@moeller-medical.com). -
Page 26: Disposal
® Vibrasat power Disposal 10 Disposal The present device contains material which must be disposed in accord- ance with environmental regulations. This device is subject to the European Directive 2012/19/EU on Waste Electric and Electronic Equipment (WEEE2). The identification plate of the device bears the symbol of the crossed through garbage bin. -
Page 27: Appendix
® Vibrasat power Appendix 11 Appendix Technical Data Artikel-Nummer: ® Vibrasat power with hand grip compl. packaged 00002246 Hand grip compl. packaged 00002327 Dimensions: Control unit (B*H*T) = 150 mm * 100 mm * 200 mm Hand grip ( ø * L ) = 55 mm * 180 mm Weight Control unit 2,80 kg... - Page 28 ® Vibrasat power Appendix Humidity 30 to 75 % rel. humidity Transport and storage conditions: Temperature 10 °C to +50 °C (14 °F to 122 °F) Humidity less than 90% rel. humidity Protection rating: Control unit IP 30 Hand grip can be steam sterilised page 28 of 38...
-
Page 29: Accessories Of The Vibrasat Power
® Vibrasat power Appendix ® Accessories of the Vibrasat power Fastening kit ® for retaining the Vibrasat power on the suction unit ® Vacusat power. Order no.: 00002283 Handgrip Ergonomic manual hand piece for ® Vibrasat suction cannulas, alu- minium anodised, natural colour Order no.: 00002254 ... - Page 30 ® Vibrasat power Appendix Liposuction cannulas with steel socket (surgical stainless steel) Vibrasat ® liposuction cannulas (3-hole cannulas) Ø = 2.5 mm, Length = 150 mm Order no.: 00001538 Ø = 3.0 mm, Length = 250 mm Order no.: 00001536 ...
- Page 31 ® Vibrasat power Appendix Liposuction cannulas with plastic socket Vibrasat ® liposuction cannulas (3-hole cannulas) Ø = 2.5 mm, Length = 150 mm Order no.: 00002859 Ø = 3.0 mm, Length = 350 mm Order no.: 00002858 ...
-
Page 32: Electromagnetic Emission
® Vibrasat power Appendix Electromagnetic emission ® The Vibrasat power is intended for the use in the electromagnetic environment as speci- ® fied below. The customer and/or operator of the Vibrasat power should ensure that the ® Vibrasat power is used in an electromagnetic environment as described below. Measurement of electromag- Compliance Electromagnetic environment - guidelines... -
Page 33: Electromagnetic Immunity
® Vibrasat power Appendix Electromagnetic immunity Electromagnetic environ- IEC 60601 test level Immunity test Compliance level ment / guidelines Floors should be from wood, ±8 kV contact dis- ±8 kV contact dis- concrete or ceramic tile. If charge Electrostatic dis- charge floors are covered with syn- charge (ESD) - Page 34 ® Vibrasat power Appendix ® The Vibrasat power complies to all test levels in accordance to IEC60601-1-2 Edition 4 (table 4 to 9). Portable RF communications equipment (radio devices) (including their ac- cessories such as antenna cables and external antennas) should not be used closer than 30 cm (or 12 inches) from the parts and cables of the Vi- ®...
- Page 35 ® Vibrasat power Appendix Electromagnetic IEC 60601-1-2 Compliance immunity test Electromagnetic environment / guidelines level test level /standard Portable and mobile RF communications equipment should be used no closer to any 150 kHz bis ® part of the Vibrasat power, including ca- 30 MHz bles, than the recommended separation dis- tance calculated from the equation applicable...
-
Page 36: Recommended Protective Distances
® Vibrasat power Appendix Recommended protective distances Refer to chapter “Electromagnetic immunity“. page 36 of 38... -
Page 37: Liposat Power Configuration
® Vibrasat power Liposat power configuration 12 Liposat power configuration ® Liposat power configuration is the medical complete system for tumescence local an- aesthesia consisting of: ® Liposat power - the medical infiltration pump for tumescence solution ® Vacusat power - medical vacuum extractor ®... - Page 38 Current revision 2020-03 O Software version 18.02.02 Order number for Instruction manual (REF) 92007181 Möller Medical GmbH Wasserkuppenstrasse 29-31 36043 Fulda, Germany Tel. +49 (0) 661 / 94 19 5 – 0 Fax +49 (0) 661 / 94 19 5 – 850 http://www.moeller-medical.com info@moeller-medical.com...








Need help?
Do you have a question about the Vibrasat power and is the answer not in the manual?
Questions and answers