Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Fresenius Medical Care T688 Series
- Page 1 Medical Treatment Chair T688 Series Instruction Manual...
-
Page 2: Table Of Contents
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series CONTENTS INDICATIONS FOR USE & GENERAL SAFETY ......2 Indications for Use ............2 General Safety .............. 2 Side Effects ..............3 Contraindications ............3 Reporting Serious Incident ..........3 OPERATING THE MEDICAL TREATMENT CHAIR .... -
Page 3: Indications For Use & General Safety
The T688 Series chair is also used to position patients for easy access by healthcare professionals. The T688 Series chair is intended to be used by patients with a weight not exceeding 200 kg (440 lbs). General Safety... -
Page 4: Side Effects
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series Side Effects None Contraindications There is no absolute contraindication for medical treatment chairs. But limitation of use in patients with history of: • Allergy to covering material of chairs •... -
Page 5: Operating The Medical Treatment Chair
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series OPERATING THE MEDICAL TREATMENT CHAIR 1. WARNING: To avoid risk of an electric shock, this chair must only be connected to supply mains with protective earth. 2. When the chair is stationary and positioned to give minimum interference to staff, ensure that all four brakes are locked by depressing the pedal on top of each castor or depressing the centre locking pedals (if fitted) on both sides of chair. - Page 6 FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series 16. To allow the patient to rise from the chair, lower the chair and return it to the upright position. (press and hold memory position #2 until in position) 17.
-
Page 7: Installing The Medical Treatment Chair
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series INSTALLING THE MEDICAL TREATMENT CHAIR Note: Transport damage, if any, should be inspected and reported immediately after delivery. No claims for transport damage will be accepted 7 days after the delivery date. -
Page 8: Battery Backup (If Fitted)
The T688 Series chair can also be supplied with an optional Seat Height Foot Control (HI/LO). Only nursing staff should operate the Height function. the Nurse hand control should be kept out of reach of the patient at the rear of the chair. - Page 9 FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series Seat Height Foot Control Page 8 of 20 IFU T688 EN V7 Apr 2021...
-
Page 10: Programming Instructions
Note: If the system does not beep twice when all of the actuators have stopped, please contact Fresenius Medical Care or your local representative for further assistance. If the chair does not respond to Process 1, please follow the instructions below (Process 2). -
Page 11: Maintenance
INSPECTION & SERVICING WARNING: No modification of this equipment is allowed. No part of the chair is user- serviceable. All servicing must be carried out by Fresenius Medical Care or authorised agent. Routine servicing, at least annually, is highly recommended in order to maintain the safe operation of the chair. -
Page 12: Fabric Standards
Flame Retardant tested to AS1530.3* UV Stable - tested to ISO 105-B02 Colour fastness to artificial light: Xenon arc fading lamp test* *NOTE: This is for Fresenius Medical Care standard supply fabric. Customer specific fabric may not meet these standards. TROUBLESHOOTING In the event the chair fails to operate correctly please take the following steps prior to contacting Fresenius Medical Care or its authorised agent. -
Page 13: Arranging A Service
ARRANGING A SERVICE In the event that the chair fails to operate correctly or to arrange a service please contact your local representative or Fresenius Medical Care Office. Note: Please quote the chair serial number ( which can be found on the Type Label attached to the chair. -
Page 14: Warranty Information
The following information outlines the warranty and Conditions of Sale for the Medical Treatment Chair 1. Fresenius Medical Care will repair or replace, at its discretion, any component or assembly, which exhibits failure or undue wear when subjected to normal use, and/or used in the manner which was intended at the time of sale. -
Page 15: Physical Description
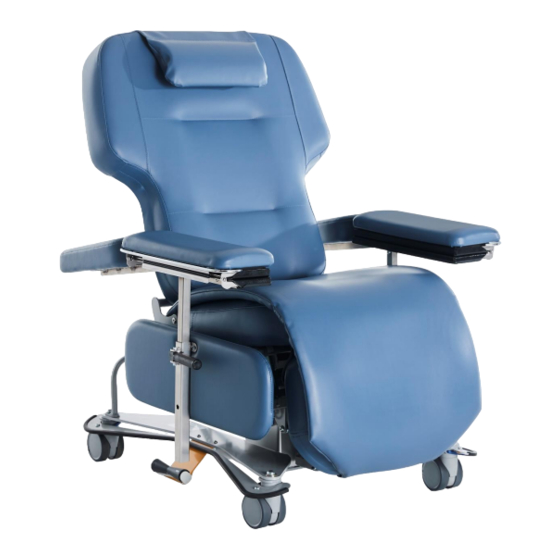
MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series PHYSICAL DESCRIPTION DESCRIPTION OF DEVICE T688 Series Medical Treatment Chair consists of: • Electrically powered chair with three (3) actuators; one (1) or two (2) hand controls (one nurse control & one optional patient... -
Page 16: Operating Conditions
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series 8.2.4 Operating Conditions 5ºC – 40ºC (41ºF - 104ºF) Temperature range 20% – 90% Relative humidity 8.2.5 Transport & Storage Conditions 5ºC – 40ºC (41ºF - 104ºF) Temperature range 20% –... -
Page 17: Key To Symbols
FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series 8.2.8 Key to Symbols Article Number/Catalog Date of manufacture Number Serial Number CE mark Manufacturer For indoor use only Storage and operating Caution, warning relative humidity 20% - 90 %... - Page 18 FRESENIUS MEDICAL CARE MEDICAL TREATMENT CHAIR INSTRUCTION MANUAL - T688 Series pressed. Seat Height Foot Control (optional) Symbol Raise the Chair Lower the Chair Authorised Representative in the European Community Fresenius Medical Care Deutschland GmbH Else-Kröner-Straße 1, 61352 Bad Homburg, Germany...
- Page 19 Guidance and manufacturer’s declaration – electromagnetic immunity The T688 Series chair is intended for use in the electromagnetic environment specified below. The customer or the user of the T688 Series chair should assure that it is used in such an environment. IMMUNITY test...
- Page 20 Guidance and manufacturer’s declaration – electromagnetic immunity The T688 Series chair is intended for use in the electromagnetic environment specified below. The customer or the user of the T688 Series chair should assure that it is used in such an environment. Electromagnetic environment –...
- Page 21 RF communications equipment and the T688 Series chair The T688 Series chair is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the [ME EQUIPMENT or ME SYSTEM] can...
- Page 22 IFU T688 EN V7 April 2021...












Need help?
Do you have a question about the T688 Series and is the answer not in the manual?
Questions and answers