Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Esaote MyLabX7VET
- Page 1 Rev. 03 July 2019 MyLabX7VET GETTING STARTED 350037400...
- Page 2 MyLab - G E T T I N G S T A R T E D...
-
Page 3: Manufacturer's Address
MyLabX7VET For US Customers: US Federal Law restricts this device to sale, distribution and use by or on the order of a physician. All the information included in this manual is relative to the following Esaote ultrasound equipments: MyLabX7VET In this manual, all the above mentioned systems are referred to as MyLab Unless specifically noted, sections of this manual pertain to all the systems. -
Page 4: Guarantee
Guarantee The information in this document is the exclusive property of Esaote S.p.A. and is reserved. Reproduction or distribution in any form is strictly prohibited. All rights reserved. All screenshots, pictures and graphics in this manual are used for descriptive purposes only and may be different from what you see on the screen or device. -
Page 5: Ec Declaration Of Conformity
EC Declaration of Conformity MyLab - G E T T I N G S T A R T E D... -
Page 6: Red Declaration Of Conformity
RED Declaration of Conformity MyLab - G E T T I N G S T A R T E D... - Page 7 MyLab - G E T T I N G S T A R T E D...
- Page 8 MyLab - G E T T I N G S T A R T E D viii...
-
Page 9: Table Of Contents
I N D E X Table of Contents Manufacturer’s Address ................i-iii Important Information ................i-iii Guarantee..................... i-iv Trade Marks....................i-iv EC Declaration of Conformity..............i-v RED Declaration of Conformity ............. i-vi 1 Introduction.................. 1-1 Safety and Standards................1-1 Getting Started ................... 1-1 Probes and Consumables.............. - Page 10 I N D E X Recommended Distances between Radiofrequency (RF) Communication Systems and MyLab..........2-6 Wireless Requirements ................2-7 3 System Overview ................3-1 About the system..................3-1 Intended Use.....................3-1 Clinical Applications and Supporting Probes ........3-2 Patient population..................3-5 Operator profile ..................3-5 Contraindications ..................3-5 System Overview..................3-5 Control Panel Assembly................3-6 Console ......................3-7 Electrical Connection ................3-8...
- Page 11 I N D E X On/Off Button .................. 5-5 Menu Button..................5-6 ETOUCH Button ................5-6 Touchscreen..................5-6 TGC Sliding ..................5-9 Information about the Screen Layout ............. 5-9 Heading Area..................5-10 Footer Area .................... 5-10 Trackball .................... 5-11 Wi-Fi ....................5-11 Archival Media..................
- Page 12 I N D E X PROBE BUTTONS Folder ............6-15 RAW DATA Folder.................6-15 KEYBOARD BUTTONS Folder ..........6-15 Security.....................6-16 Licenses Manager ...................6-16 License Activation ................6-16 Import/Export Menu................6-18 EXPORT Folder ................6-18 IMPORT Folder ................6-19 System Info .....................6-20 7 Performing an Exam..............7-1 Starting an Exam ..................7-1 Entering Patient and Application data..........7-3 Filling the Patient ID screen .............7-3 Retrieving data from archive.............7-3...
- Page 13 I N D E X Software ....................9-4 Biometry....................9-4 Keyboard ....................9-5 Dimensions....................9-5 Weight ....................... 9-5 IP Grade....................9-5 Power supply.................... 9-5 Batteries..................... 9-5 Power Cables ....................9-6 Opti-light ....................9-7 Operating Requirements ................ 9-7 Storage requirements................9-7 Probe Storage Requirements ..............
- Page 14 I N D E X MyLab - G E T T I N G S T A R T E D...
-
Page 15: Introduction
MyLabX7VET family, or when the contents are common to the other MyLab ultrasound systems belonging to the Esaote platform. MyLab Safety and Standards The Safety and Standard manual contains information about the patient's and operator's safety. -
Page 16: Advanced Operations Manual
NOTE Specifications subject to change without notice. Information might refer to products or modalities not yet approved in all countries. Product images are for illustrative purposes only. For further details, please contact your Esaote sales representative. This manual refers to... -
Page 17: Intended Audience
Before reading these instructions for use, you need to be familiar with ultrasound techniques. Sonography training and clinical procedures are not included here. Veterinary Use Esaote offers dedicated software intended for veterinary use, providing specific probes and measurements. The application of our imaging diagnostic devices to other species/ WARNING practices as well as for conventional species is done under veterinary specialist's exclusive responsibility, knowledge and belief. - Page 18 I N T R O D U C T I O N Special attention should be dedicated to intra-cavitary probes (e.g. vaginal, rectal or oesophageal probes). They should be cleaned according to established protocols (AIUM guidelines for cleaning probes) and should not be used if there is noticeable self-heating of the probe when operating in air.
-
Page 19: Mylab Use
Use of the product for the purposes other than those intended and expressly stated by Esaote, as well as incorrect use or operation, may relieve Esaote or its agents from all or some responsibility for resultant noncompliance, damage, or injury. -
Page 20: Manufacturer's Responsibility
In this manual NOTE points out information of special interest but not NOTE related to risks for patient, operator or device. Manufacturer’s Responsibility Esaote is responsible for the safety, reliability and functioning of this product only if: the user follows all the instructions contained in the system manuals for the use and the maintenance of this system;... -
Page 21: Maintainability Time
Esaote qualified personnel, using original Esaote spare parts. When approaching the seven (7) years limit from the purchase date, it is recommended to contact Esaote Service or to visit Esaote web site (www.esaote.com), to get updated information on the product’s end of life and/or to agree on the most suitable solution for its safe disposal. -
Page 22: Proprietary Rights
For any software updates and/or upgrades installed on the Equipment after installation, the terms herein shall apply in full. License Rights and Limitations With this license, Esaote S.p.A. grants the end user the right to use the on the supplied SOFTWARE... -
Page 23: Third Part Software
I N T R O D U C T I O N Third Part Software Esaote software uses parts of the 7-Zip program. The 7-Zip is licensed under the GNU LGPL license; the source code can be found in www.7-zip.org. - Page 24 I N T R O D U C T I O N PRODUCT TRACEABILITY FORM To: ESAOTE S.p.A. Quality Assurance Department Via Enrico Melen, 77 16152, Genova, Italy [or associate company] [or authorized distributor] Esaote system/device name:..................REF..........................Serial Number (SN):....................
-
Page 25: Vigilance System
Esaote central plants, or one of our subsidiaries, or one of our official distributors immediately through the following form, or through a communication reporting the same data contained in this form. - Page 26 I N T R O D U C T I O N POST-MARKET SURVEILLANCE FORM ACCIDENT REPORT FORM To: ESAOTE S.p.A. Quality Assurance Department Via Enrico Melen, 77 16152, Genova, Italy [or associate company] [or authorized distributor] ESAOTE system/device name:................
-
Page 27: Additional Information On Safety
Chapter 2. Additional Information on Safety This chapter provides additional information on safety specifically for MyLab products. Please read the “Safety and Standards” manual carefully for a complete overview of all safety aspects of products. MyLab Environmental Safety Special waste The system contains lithium-ion batteries. -
Page 28: Electromagnetic Compatibility
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y Electromagnetic Compatibility This system was designed for use in the electromagnetic environments declared in the tables below, in compliance with standard IEC 60601-1- 2:2014 (4 edition). -
Page 29: Essential Performance
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y Use of accessories, transducers and cables other than those specified or WARNING provided by the manufacturer of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this... -
Page 30: Electromagnetic Immunity For All Medical Equipment
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y Electromagnetic Immunity for All Medical Equipment MyLab system is intended for use in the electromagnetic environment specified below. The MyLab customer or the user of the system should assure that it is used in such an environment. -
Page 31: Electromagnetic Immunity For Medical Equipment Not Life Supporting
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y MyLab system is intended for use in the electromagnetic environment specified below. The MyLab customer or the user of the system should assure that it is used in such an environment. -
Page 32: Recommended Distances Between Radiofrequency (Rf) Communication Systems And Mylab
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y Recommended Distances between Radiofrequency (RF) Communication Systems and MyLab As stated in the “Safety and Standards” manual, it is recommended not to use radiofrequency (RF) transmission systems near the ultrasound system. -
Page 33: Wireless Requirements
A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y Wireless Requirements is equipped with built-in wireless capability. MyLab When wireless is active, the operator should make sure to stay at a minimum distance of 20 cm from the rear of the equipment. - Page 34 A D D I T I O N A L I N F O R M A T I O N O N S A F E T Y MyLab - G E T T I N G S T A R T E D 2 - 8...
-
Page 35: System Overview
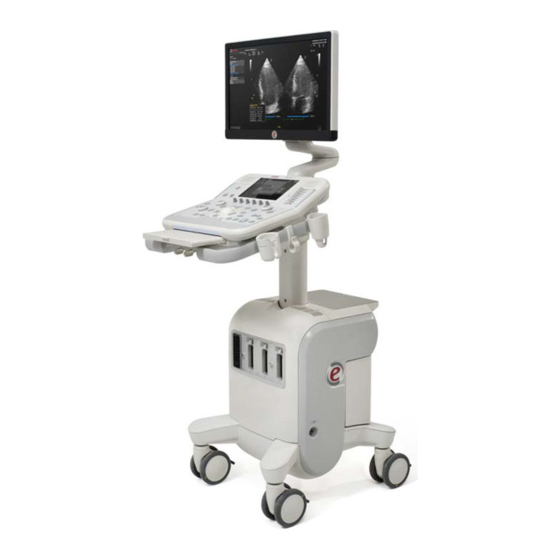
Chapter 3. System Overview is a professional, innovative and versatile real-time high- MyLabX7VET resolution ultrasound systems. The wide range of probes makes it suitable for many clinical applications. is based on a mainframe easily movable platform. MyLabX7VET MyLabX7VET has four swiveling wheels, it has a range of height adjustments for one-time installation, the main screen can be easily moved due to an optional articulated arm. -
Page 36: Clinical Applications And Supporting Probes
The ultrasonic medical diagnostic equipment is intended to be connected to mechanical and electronic ultrasound probes (convex array, linear array and phased array) and Doppler probes. Clinical Applications and Supporting Probes A variety of ultrasound probes can be connected to MyLabX7VET AC2541 including biopsy capabilities IH 6-18 ... - Page 37 S Y S T E M O V E R V I E W Table 3-1: Available probes and applications Probes Application Abdominal bovine AC2541, IL 4-13, IOT342, L 3-11, mC 3-11, P 1-5, SB2C41, SC3123, SI2C41, SL3116 Abdominal canine AC2541, IH 6-18, IL 4-13, IOT342, L 3-11, L 4-15, mC 3-11, P 1- 5, P 2-9, P2 5-13, SB2C41, SC3123, SI2C41, SL1543, SL2325, SL3116...
-
Page 38: Contraindications
System applications are dependent on your system configuration, NOTE transducer and exam type. Not all applications are approved in all Countries. Please refer to your Esaote local representative for further information. Contraindications is not intended for: MyLabX7VET ... -
Page 39: Control Panel Assembly
S Y S T E M O V E R V I E W Fig. 3-1: MyLabX7 System Control Panel Assembly The control panel assembly includes the system handle, all system controls, the touchscreen, the loud speakers, the probes, gel and ECG cables holders. The LCD is installed on top of the assembly. -
Page 40: Console
S Y S T E M O V E R V I E W Pull out Qwerty A pull out alphanumeric keyboard is placed at the base of the control panel. Keyboard The keyboard is mounted on a sliding drawer: just put it to extract the keyboard. -
Page 41: Electrical Connection
S Y S T E M O V E R V I E W Headphone and On the left side of the console, just below the USB ports, there are two (2) Microphone connectors: one connector for the headphone and one connector for the microphone. -
Page 42: Probe Connections
S Y S T E M O V E R V I E W Procedure 1. Open the rear door. 2. Plug in the power cord. 3. Close the rear door by letting the cord go through the lower slot. 4. -
Page 43: Control Panel Assembly Orientation
S Y S T E M O V E R V I E W Probes with big connector Make sure that the connector-securing device is in the “OPEN” position, align the pins of the two connectors and carefully attach the probe. To secure it, move the securing device to its “LOCK”... - Page 44 S Y S T E M O V E R V I E W The orientation lever, located on the left, allows lateral rotation of the assembly. The lifting lever, located on the right, allows to lift or lower the control panel. Symbol Orientation lever Lifting lever...
-
Page 45: Batteries
MyLab batteries. A fully charged battery ensures more than one hour of scanning. The battery pack is installed by Esaote personnel. This person will be NOTE responsible for its installation and for ensuring that the system is working properly. -
Page 46: First Use
S Y S T E M O V E R V I E W A system which has not been used for a month needs to be charged before using it with the battery. Charge and discharge the battery only when the environment temperature CAUTION is between 15°C and 30°C. -
Page 47: Battery Lifetime
Esaote recommends to replace the battery pack every three years. The battery pack has to be replaced by Esaote personnel. This person will NOTE be responsible for ensuring that the system is working properly. -
Page 48: Power Supply Error Messages
Switch on again and check whether the MyLab message is still present. If the problem persists, contact Esaote personnel. Error #4 This error indicates that at least one of the batteries has reached the maximum temperature allowed for its working conditions. - Page 49 Verify that nothing is blocking the fan functioning, especially on the rear panel. Should the problem persist, contact the Esaote Service department. Error #7 This error indicates that a fault of the internal voltages occurs. The system displays the following message: Error #7: problem with internal voltage.
- Page 50 S Y S T E M O V E R V I E W MyLab - G E T T I N G S T A R T E D 3 - 16...
-
Page 51: Preparing The System
Chapter 4. Preparing the System The system will be installed by Esaote personnel. Esaote personnel will be responsible for opening the packaging and ensuring that the system is correctly programmed and operational. The information and procedure provided in this chapter will guide to prepare the system for use. -
Page 52: Connecting Peripherals
MyLab usually already mounted and connected. The first mounting and connecting will usually be performed by an Esaote technician. Esaote suggests to contact its Service representative to install any auxiliary NOTE device. Before installing the peripheral devices, make sure that the system is switched off and unplug the power cable from the mains outlet. -
Page 53: Medical Environments
P R E P A R I N G T H E S Y S T E M Furthermore, all configurations shall comply with the requirements for medical electrical systems (see IEC 60601-1-1 or clause 16 of the 3rd Edition of IEC 60601-1, respectively). - Page 54 B or area C connected by cable (USB, HDMI,...) - Auxiliary device must be powered through a safety insulation transformer complying to IEC 60601. Auxiliary Devices must be approved by Esaote. Auxiliary Devices must also NOTE comply with EN 60601-1-2 safety standard and subsequent amendments or the electromagnetic compatibility.
- Page 55 The system must be powered so to satisfy the electrical safety requirements, WARNING as specified in the “Safety and Standards” manual. Esaote recommends running a current leakage (patient and environment) test when installing in order to check whether the applicable limits of standard EN60601-1 are not being surpassed.
-
Page 56: B/W Thermal Medical Usb Printer Housing
P R E P A R I N G T H E S Y S T E M B/W Thermal Medical USB Printer housing Those kinds of printers can be hosted in the lateral storage area. Procedure 1. Open the rear door. 2. -
Page 57: Auxiliary Monitor
Mechanical damage may MyLab occur if the drive falls down. The External Slim DVD Writer kit supplied by Esaote includes a Velcro tape to easily and securely fix it to console. Stick the tape both on the MyLab... -
Page 58: Moving And Transporting The System
P R E P A R I N G T H E S Y S T E M only if powered by their external power supply through a medical insulation transformer. Moving and Transporting the System is provided with wheels and handle to allow the user to easily move the MyLab device. - Page 59 P R E P A R I N G T H E S Y S T E M Make sure that the probes are locked and the probe cables are properly hanged in the cable hooks while moving the system. The handles on the keyboard cannot be used to lift the system.
-
Page 60: Mylab Quick Displacement
P R E P A R I N G T H E S Y S T E M Fig. 4-4: Transporting Configuration Protect the screen orientable arm so that no lateral movements are possible (for example with film). Use the brakes to lock the system. - Page 61 P R E P A R I N G T H E S Y S T E M The touchscreen displays a message indicating the residual time after which will automatically start the shut down procedure. MyLab Fully charged batteries ensure more than half an hour of self-powering. NOTE Move the system to the working position and connect it to the mains: the LCD display is automatically turned on and the system is ready for its use.
- Page 62 P R E P A R I N G T H E S Y S T E M MyLab - G E T T I N G S T A R T E D 4 - 12...
-
Page 63: Using The System
Chapter 5. Using the System This chapter provides a brief description of the system controls. Refer to the “Advanced Operations” Manual for further detailed information. Connecting the system to the mains The power plug and the electrical main switch are located on the rear-bottom of the system. -
Page 64: System Controls
U S I N G T H E S Y S T E M 6. Connect the network and other cables from the system to the appropriate wall plugs. 7. Plug the cable to a reliable grounding power outlet to assure adequate grounding. - Page 65 U S I N G T H E S Y S T E M Table 5-1: Exam Control Buttons Button Description Gives access, at any time, to the archived data. ARCHI VE Closes the current exam archiving the patient’s data and producing EN D EXAM a report on the exam.
-
Page 66: Trackball
U S I N G T H E S Y S T E M Button Description Activates three dimension features; further details on how to use 3D/4D them are described in the “Advanced Operations” manual In B-Mode or CFM, this button allows to interactively activate the LI N E UPDATE cursor or disable it to select the M-Mode or Doppler line. -
Page 67: Touchscreen Section
U S I N G T H E S Y S T E M The cursor function is indicated below the image. When several cursors are present on the screen, switches between the active cursors. Yellow ACTI O N indicates active cursor function while white next cursor function. Mouse Mode In its mouse mode, the trackball can be used to move a pointer on the screen, to access to the thumbnails of the images, displayed on the right side of the... -
Page 68: Menu Button
U S I N G T H E S Y S T E M Optional Batteries When is equipped with optional batteries, the same button places the MyLab system in stand-by, partially shutting it down: in this case the initialization phase at start up is significantly reduced. - Page 69 U S I N G T H E S Y S T E M Exam Panel Layout This layout is used for standard exam functions necessary to perform the exam. The touchscreen is organized in four main areas. Fig. 5-1: CFM touchscreen 1.
- Page 70 U S I N G T H E S Y S T E M Table 5-4: Touchscreen key status Active Key with Disabled Key Active Key Selected Key Submenu Gray text on dark White text on light As Active Key with Blue text on dark gray background gray background...
-
Page 71: Tgc Sliding
U S I N G T H E S Y S T E M Alphanumeric The alphanumeric keyboard is based on the QWERTY standard. The Keyboard Layout alphanumeric keys are used to enter text data in the enabled windows. The Caps Lock key sets the keyboard to upper case characters. -
Page 72: Heading Area
U S I N G T H E S Y S T E M Controls on the Screen Layout are indicated in the operator manuals with , while strings and fields are indicated B O L D B L A C K C A P I T A L L E T T E R S with NORMAL BLACK CAPITAL LETTERS Symbol on Screen... -
Page 73: Battery
U S I N G T H E S Y S T E M Archival Media Archival media are shown on the left. The icon is shown crossed out whenever there are management problems involving the specific archival system. For more details on data archival, consult the relevant section on the “Advanced Operations”... -
Page 74: Image Area
U S I N G T H E S Y S T E M Image Area The visualization of the image depends on various factors such as the active mode, the selected application and the probe. The following figure shows the elements in the image area that are independent of these factors. -
Page 75: Machine Parameters
U S I N G T H E S Y S T E M Machine Parameters Table 5-6: Imaging Parameters Displayed Parameter Description format Imaging or TEI (Tissue Enhancement Imaging) mode: General, Resolution or Penetration (L: Low, H: High) Imaging gain (Min,%, Max) Auto Adjust nn mm Depth... -
Page 76: Thumbnails Area
U S I N G T H E S Y S T E M Table 5-8: Doppler Parameters Displayed Parameter Description format nnn MHz Doppler frequency or TV (Tissue Velocity) frequency when enabled Doppler gain (Min,%, Max) nnn kHz Pulse Repetition Frequency Dynamic range / Rejection nnn Hz Wall filter... -
Page 77: Customizing The System
Chapter 6. Customizing the System can be customized to increase efficiency and streamline your work- MyLab flow. You can do the following: Create preset designed specifically for the exams you perform. Change system settings to reflect your needs. ... - Page 78 C U S T O M I Z I N G T H E S Y S T E M The menu is organized in three areas: Clinical Configuration, the upper area shows all options relating to Clinical Settings or Presets, ...
- Page 79 C U S T O M I Z I N G T H E S Y S T E M System Settings System Settings define the ’s parameters related to a specific system MyLab profile: Profile Manager, DICOM configuration, ...
-
Page 80: Generic Configuration Procedure
C U S T O M I Z I N G T H E S Y S T E M Generic Configuration Procedure Once accessed the configuration screen for the parameter you want to set, a common set of commands is available and a common setting procedure can be used. -
Page 81: Clinical Configurations
C U S T O M I Z I N G T H E S Y S T E M Clinical Configurations This chapter explains how to set many options. For configurations not MyLab described here refer to relevant chapters in the “Advanced Operations” manual. Real Time Preset A Preset is a group of settings that optimizes the system for a specific type of exam. -
Page 82: Creating A New Preset From Real-Time
C U S T O M I Z I N G T H E S Y S T E M Creating a new preset from Real-Time Procedure To create a new preset or modify an existing one: Adjust the real time image as desired in all modes (2D, CFM and Doppler). - Page 83 C U S T O M I Z I N G T H E S Y S T E M on the right the menu to record the macro and to edit the customized buttons, on the bottom the fields where customized touchscreens are named and described.
-
Page 84: System Settings
C U S T O M I Z I N G T H E S Y S T E M button adds a new tab that will be automatically displayed. Place N E W T A B the cursor on the tab and press to change its name, using the EN TER alphanumeric keyboard to edit it. -
Page 85: Corrupted System Profile
(no component has to be without any setting). Should this not solve the problem, contact Esaote personnel. Center ID Center ID allows to set the center name displayed in the Heading Area of the screen and the center information shown in the report. -
Page 86: General Setup
C U S T O M I Z I N G T H E S Y S T E M General Setup The menu is organized in internal folders, selectable using the tabs displayed on the top of the menu. Fig. -
Page 87: Measure Units Folder
C U S T O M I Z I N G T H E S Y S T E M Set Time Using the keyboard set the time. Time Format The time format is available on a 24 or 12 hour basis. In the 12 hour option, the time is shown as AM and PM. -
Page 88: Control Panel Folder
C U S T O M I Z I N G T H E S Y S T E M CONTROL PANEL Folder The table below lists and explains the available fields and the corresponding actions. Table 6-3: Control Panel Folder Fields Action TRACKBALL SPEED... -
Page 89: Field Shutdown Type
If a virus is found, it is advisable to switch off the , disconnect it from MyLab the data network and call the Esaote service, which will check for the presence of a virus and restore the system. Field AVAILABLE QWERTIES... -
Page 90: Cine Mode Folder
C U S T O M I Z I N G T H E S Y S T E M Fig. 6-4: Qwerty keyboard CINE MODE Folder When set, the options AUTOMATIC PLAY TRACE AUTOMATIC PLAY respectively allow to review the stored images and the trace in cine mode when is pressed. -
Page 91: Footswitch Folder
C U S T O M I Z I N G T H E S Y S T E M Fields Action AUTO BUTTON SETUP Sets the action after pressure: AUTOADJUST AUTO automatically adjust the B-Mode image), ECFM automatically optimize CFM image) or of them. -
Page 92: Security
C U S T O M I Z I N G T H E S Y S T E M When the buttons are associated to save image or save clip, they NOTE will be named further in this manual as respectively. - Page 93 C U S T O M I Z I N G T H E S Y S T E M License Activation To activate a new license, type the license number in the field and press KEYS to confirm. If the number is correct, the status changes to V E R I F Y PERMANENT All license fields are not case sensitive with the exception of the CrystaLine...
-
Page 94: Import/Export Menu
C U S T O M I Z I N G T H E S Y S T E M Import/Export Menu The menu is organized with internal folders, selectable using the tabs displayed on the top of the menu. Fig. -
Page 95: Import Folder
C U S T O M I Z I N G T H E S Y S T E M customized SAVING OPTIONS customized center configuration ( CENTER ID customized export settings; MULTIMEDIA customized configurations; NETWORK ... -
Page 96: System Info
ENCRYPTION MODE Encryption Mode Encryption allows to preserve health data storage confidentiality. Encryption can be performed by Esaote Service personnel only. Encryption can be applied to the internal hard disk and to one or more external USB memory devices. At the end of encryption a recovery key will be given to you. The recovery key is stored on USB pen drive, or on file, or by printing it. -
Page 97: Performing An Exam
Chapter 7. Performing an Exam This chapter describes the procedures commonly used in performing patient exams with . These procedures include entering patient and application MyLab data, acquiring images, making measurements and calculations; annotating and reviewing images. Read the “Safety and Standards” manual carefully: all the safety characteristics, cautions and warnings listed there apply to all exams. - Page 98 P E R F O R M I N G A N E X A M Fig. 7-1: Patient ID screen Fig. 7-2: Probe, Application, Preset touchscreen Starting exam The steps to be followed to start an exam are: procedure 1.
-
Page 99: Entering Patient And Application Data
P E R F O R M I N G A N E X A M Entering Patient and Application data There are two ways to enter patient data: Filling the Patient ID screen, Retrieving existing data from archive. Filling the Patient ID screen The Patient ID screen is used to enter patient data and application data, when applicable. -
Page 100: Selecting Probe
P E R F O R M I N G A N E X A M At any time during the exam, Patient Data can be viewed and modified by PATIENT ID pressing PATIENT ID Do not use to start a new exam of a new patient as it will update WARNING existing patient’s data with new entries. -
Page 101: Selecting Application
P E R F O R M I N G A N E X A M Selecting Application When a probe has been selected, on the middle of the touchscreen all the application available with the selected probe are displayed. Tap the name of the desired application to set it. -
Page 102: Performing The Exam
P E R F O R M I N G A N E X A M Performing the Exam offers a set of imaging modes to cover a variety of imaging needs. By MyLab pressing the different mode buttons the specific mode is activated in real time. -
Page 103: Reviewing Images
P E R F O R M I N G A N E X A M Reviewing Images EXAM REVIEW During the exam, taping enables reviewing of the saved images and sequences, and the trackball automatically changes to pointer mode, allowing you to scroll through the thumbnails and select the item to be reviewed. - Page 104 P E R F O R M I N G A N E X A M At power-up, the system prompts the operator to archive the last exam NOTE performed if the system was switched off without first closing the exam in progress.
-
Page 105: System Maintenance
Chapter 8. System Maintenance To ensure that operates over time at its maximum efficiency, Esaote MyLab recommends to perform maintenance procedures regularly. Maintenance procedures should be performed both by the user itself and by Esaote authorized service personnel. The maintenance operations and schedule are provided in the table below. -
Page 106: Veterinary Applications
Refer to “Probes and Consumables” manual for periodic inspections for probes and cleaning instructions. Veterinary Applications Due to the specificity of the veterinary working place, Esaote recommends WARNING that the ultrasound devices used in veterinary applications should be thoroughly cleaned at least once every 6 months. Please contact your Esaote distributor or dealer for further help in this matter. -
Page 107: Cleaning Operations
S Y S T E M M A I N T E N A N C E Cleaning Operations Periodic cleaning of the system and any connected devices is important. In the event of poor maintenance, dust and dirt can compromise the reliability and performance of and connected devices. -
Page 108: Cleaning Control Panel And System
S Y S T E M M A I N T E N A N C E Check the instructions supplied by the manufacturer of the cleaning agents for possible stricter limitation. Do not use hot cleaning agents for cleaning the equipment. -
Page 109: Cleaning Probe And Gel Holders
S Y S T E M M A I N T E N A N C E Invisible laser radiation. Do not view directly with optical instruments. WARNING Class 1M laser products. Do not remove the ball from the socket. Do not try to disassemble the trackball during the cleaning of the WARNING removable sealing ring. -
Page 110: Cleaning The Lcd Screen
S Y S T E M M A I N T E N A N C E Cleaning the LCD Screen To clean the LCD use a soft dry cloth, lightly rubbing the display surface to remove dust and other particulate matter. If necessary, apply a small amount of ammonia- free glass cleaner onto a clean, soft cloth and then wipe the surface. -
Page 111: Technical Specifications
Licenses enable specific functions of the system, they are linked to the system serial number and are, therefore, unique. They should be carefully stored. The system is delivered by Esaote, with the licenses already installed. Additional features can be added buying the related license. - Page 112 T E C H N I C A L S P E C I F I C A T I O N S Applications systems can be equipped with the following application licenses. MyLab Table 9-1: Application licenses Licence Application Features General Imaging Abdominal Canine, Abdominal...
- Page 113 T E C H N I C A L S P E C I F I C A T I O N S Licence Feature description Multi-modality & Multi-modality management Dicom Q/R Multi-modality DICOM Query/Retrieve ElaXto ElaXto allows you to perform elastosonographic analysis of the tissues ElaXto Measures It enables measurements in elastosonography...
-
Page 114: Technical Characteristics
XView+ pixel level, eliminating speckle and noise artefacts. a. Refer to www.esaote.com for further details on supported DICOM classes. b. Refer to the corresponding Sales Area manager for further information. Features, probes and applications availability is dependent on your system NOTE configuration. -
Page 115: Connectivity
Annotations, bodymarks Keyboard Height adjustable control panel Control panel: • Potentiometers for TGC 1. Refer to www.esaote.com for further details. MyLab - G E T T I N G S T A R T E D 9 - 5... -
Page 116: Dimensions
T E C H N I C A L S P E C I F I C A T I O N S • Encoders for general gains • Keys for modes, peripherals management and controls Reconfigurable touchscreen LCD ... -
Page 117: Power Cables
T E C H N I C A L S P E C I F I C A T I O N S Power Cables Table 9-3: Power Cables Connector Plug Type Cord Type Length Italy EN60320/C13 I/3G CEI 23-50 H05VVF3G 4,5 m Chile... -
Page 118: Opti-Light
T E C H N I C A L S P E C I F I C A T I O N S Connector Plug Type Cord Type Length Denmark EN60320/C13 DK3/HGA H05VVF3G 4,5 m SB 107-2D1 DK2- Section 1 mm 3 conductors 10A-250V South Africa... -
Page 119: Standards
T E C H N I C A L S P E C I F I C A T I O N S Standards Table 9-4: Standards Standard Title IEC 60601-1:2012 (Ed.3.1) Medical electrical equipment - Part 1: General EN 60601-1:2006 + CORR. 1 requirements for basic safety and essential (2006) + CORR. - Page 120 T E C H N I C A L S P E C I F I C A T I O N S Standard Title AIUM/NEMA UD-3:2004 Standard for Real Time Display of Thermal and (R2009) Mechanical Acoustic Output Indices on Diagnostic Ultrasound Equipment.
















Need help?
Do you have a question about the MyLabX7VET and is the answer not in the manual?
Questions and answers