Table of Contents
Advertisement
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for GE GEH-ECG 1200
- Page 1 User Guide 2102322-111 REV 1 NOTE: The information in this manual only applies to the product GEH-ECG 1200. Due to continuing product innovation, specifications in this manual are subject to change without notice. © 2019 General Electric Company. All rights reserved...
- Page 2 Telephone: +49 (0) 6122-70524-0 Faximile: +49 (0) 6122-70524-14 E-Mail: info@norav.com 2797 GEH-ECG 1200 Recorder conforms to MDD 93/42/EEC and carry the mark accordingly. Federal Law restricts this device to sale by or on the order of a licensed physician or healthcare provider Caution...
-
Page 3: Table Of Contents
Symbols Table of contents Symbols ................................4 Device Labels ..............................4 Warning and Precautions ........................... 5 Controls and Indicators ............................6 Battery Assembly ............................... 7 Belt Strap Assembly ............................8 Patient Leadwires Assembly ........................... 9 Electrode Application ............................8 Maintenance and Cleaning ..........................10 Storage ................................ -
Page 4: Symbols
Symbols Symbols Symbol Definition Warnings call attention to possible hazards involving potential damage or injury to persons. Cautions refer to practices necessary to protect against potential damage or loss to equipment. Pay careful attention to instructions. Notes provide pertinent information to help obtain optimum performance from the device or signify an important step or procedure that requires special attention. -
Page 5: Warning And Precautions
When using GEH-ECG 1200 in combination with any other equipment, refer to a qualified service technician for correct handling. •... -
Page 6: Controls And Indicators
Use only with battery compartment of patient unit 1200W is closed. Caution • The GEH-ECG 1200 complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: This device may not cause harmful interference and ... -
Page 7: Battery Assembly
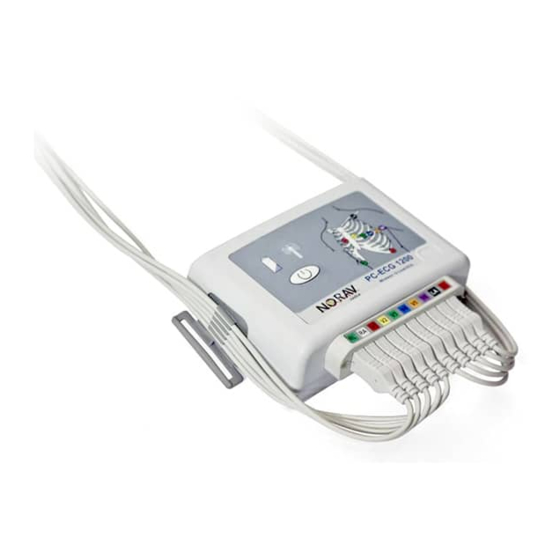
Battery Assembly Battery Assembly (1) Insert the first battery and slide it to the right. (2) Insert the second battery and push it to the left and down with the same movement. (3) Place the battery cover. (4) Push battery cover down until get click. •... -
Page 8: Belt Strap Assembly
Belt Strap Assembly Belt Strap Assembly 1. Take the end of the strap (ensuring there are no twists present), thread the strap via strap holders of the 1200W. 2. Take the end of the strap and the end of the buckle with center bar and then thread the strap from the bottom side of the buckle up between the main body of the buckle and the innermost side of the center bar. -
Page 9: Patient Leadwires Assembly
Patient Leadwires Assembly Attaching the Electrodes • Attach the leads to the electrodes before placing them on the patient. • Apply the electrodes by peeling them, one at a time, from the protective backing and sticking them firmly to the patient’s skin. •... -
Page 10: Maintenance And Cleaning
Maintenance and Cleaning Maintenance and Cleaning Remove the battery before cleaning the 1200W recorder. To clean the recorder unit and ECG lead wires, wipe with a dry cloth or a cloth soaked with regular household cleaner diluted with water. Take care to prevent chemicals\liquids from entering the connectors or internal part of the recorder. The battery contacts should not come in contact with soap or water. -
Page 11: Service
Service Service If there is a problem with the 1200W recorder, review the Troubleshooting section for a listing of problems and solutions. If additional assistance is required, contact customer support via phone, fax or e-mail listed in this manual. Call customer support before returning a recorder to make shipping arrangements. All repairs on products under warranty must be performed or approved by Norav Medical. -
Page 12: Ecg Cables And Accessories
ECG Cables and Accessories ECG Cables and Accessories Accessories Part Number Leadwires with Snaps IEC 2102322-011 Leadwires with Snaps AHA 2102322-012 Leadwires with Clips IEC 2102322-013 Leadwires with Clips AHA 2102322-014 Black belt for 1200W acquisition unit 2102322-015 USB cable 3m for 1200WR Receiver 2102322-053 BNC cable 3m for 1200WR Receiver 2102322-054... -
Page 13: Electromagnetic Emissions And Immunity Information
Electromagnetic Emissions and Immunity Information Electromagnetic Emissions and Immunity Information Refer to the following tables for specific information regarding NR recorder compliance to IEC 60601-1-2. Table 1: Electromagnetic Emissions Emissions Test Compliance Electromagnetic Environment—Guidance This device is intended for use in the electromagnetic environment specified below. The customer and/or user of this device should ensure that it is used in such an environment. - Page 14 Electromagnetic Emissions and Immunity Information radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the device is used exceeds the applicable RF compliance level above, the device should be observed to verify normal operation.
- Page 15 Electromagnetic Emissions and Immunity Information 2102322-111 REV 1 Page 15...
- Page 16 www.gehealthcare.com...











Need help?
Do you have a question about the GEH-ECG 1200 and is the answer not in the manual?
Questions and answers