Bowa ERGO 300 Information For Use
Morcellator
Hide thumbs
Also See for ERGO 300:
- Operating manual (48 pages) ,
- Instructions for use manual (39 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Bowa ERGO 300
- Page 1 Information for use ERGO 300 – Morcellator 12930 - S0 EN...
- Page 2 Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 3: Table Of Contents
Replacing the control unit fuse Safety inspections Malfunction and troubleshooting Fault messages on the display Spare parts list with order number Information on disposal Appendix Electromagnetic Compatibility (EMC) Declaration of Conformity Manufacturer and service points Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 4: General Information
Principle The ERGO 300 - Morcellator is a control unit for the Nouvag Morcellator for use in morcellation and extraction of tissue as well as for myoma removal at laparoscopic interventions. A rotating cylindrical tube with a blade at the distal end is inserted through an abdominal cavity that has been enlarged by an Insufflator. -
Page 5: Medical Application
• If any parts are damaged or missing, contact the Nouvag agent from which the device was ordered or Nouvag AG. Immediate inspection of the ERGO 300 - Morcellator - motor system set (REF 3287bow): 1. Control unit (905-001) 7. Electronic motor 21 (905-002) 2. -
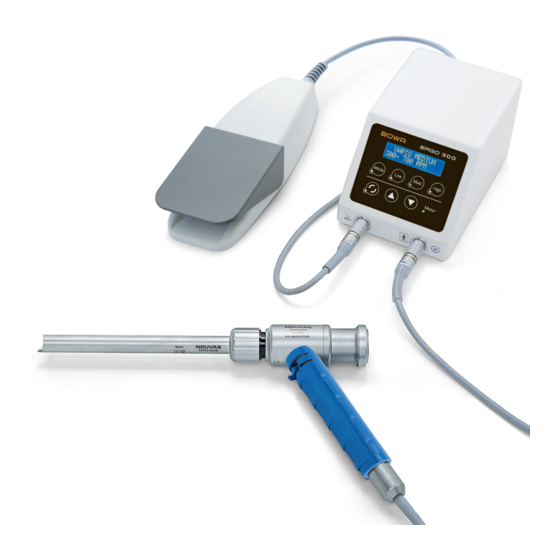
Page 6: Scope Of Delivery, Accessories And Spare Parts
Description Quantity 905-000 ERGO 300 – Morcellator set comprises the following products................1 905-001 ERGO 300 Morcellator control unit, device connecting cable 3 m, country specific ............1 905-003 Vario pedal; IPX8; connecting cable 3 m .............................1 905-002 Electronic motor 21, 3 m motor cable ............................1 905-004 Morcellator gear unit ..................................1... -
Page 7: Technical Data: Ergo 300 - Morcellator
Warranty Purchasing the ERGO 300 - Morcellator entitles you to a 1-year warranty. If you return the warranty card for registration within four weeks of the date of purchase, warranty coverage will be extended for a further 6 months. Improper use or repair, or failure to observe these instructions, will relieve us from any obligation arising from warranty provisions or other claims. -
Page 8: Explanation Of Symbols
Protected against continuous submersion in water. Manufacturer European authorized representative Dangerous goods aerosol sprays: Dangerous goods aerosol sprays: Warning! Extremely flamable NouvaClean/NouvaOil NouvaClean/NouvaOil Dangerous goods aerosol spray: Protective ground Environmentally hazardous NouvaClean/NouvaOil Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 9: Safety Information
The use of (RF) Radio Frequency emitting devices and equipment as well as the occurrence of negative environmental factors in the close area of the ERGO 300 - Morcellator may cause unexpected or adverse operation. The connection or the placing of other devices in close vicinity is not allowed. -
Page 10: Essential Requirements
Disregarding the guidelines on intermittent operation (1 min on, 1 min off for 8 cycles) may cause burns at contact with the gear unit. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 11: Device Overview
4. Motor 4. Seal unit with membrane screw 5. Handpiece holder 5. Thread for membrane screw 6. Motor connection plug 6. Membrane screw 7. Seal unit thread 8. Motor connection coupling Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 12 5. Main body 6. Nose for tissue protection Grasping forceps 1. Mouth insert 2. Gripper mouth 3. Tube shaft 4. Fixed leg Handle 5. Flexible leg 6. Locking device 7. Spring component Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 13: System-Chart, Ergo 300 - Morcellator
Seal adapter Small sealing unit for Ø 12/15 mm Handle Option 3 Protection sleeve with Ø 12 mm Myoma drill Seal adapter Small sealing unit for Ø 12 to 15 mm Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 14: Before Use
Before use The ERGO 300 – Morcellator and all required accessories and instruments must be placed on a level nonslip surface. Do not allow the operating range of the device with cable to be restricted by limiting factors. The display, keypad and indicator lights must be fully visible at all times. -
Page 15: Mounting The Morcellator Set With Protective Tube (According To Option 1)
Push the protective tube over the distal end of the cutting tube. The guide pin of the protective tube must be in the distal slot of the gear unit. Lock the protective tube in position with a click. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 16 (3) and screw the membrane fixing ring (7) onto the roof-shaped seal fixing ring (6). Now screw the entire sealing unit (8) onto the gear unit (9). Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
- Page 17 Plug the pedal device connection plug into the pedal connection of the control unit. The plug must lock in position. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 18: Assembly Of The Morcellator Set With Trocar Cannula (According To Option 2)
• The sealing flap and the seal must not be damaged. Risk of injury Select blade. Make sure that the cutting edge of the blade is sharp and undamaged (e.g. no cracks or burrs). Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 19 7. Screw the membrane screw (6) into the thread of the seal holder (3) to the stop and connect the seal unit to the gear unit. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
- Page 20 Plug the pedal device connection plug into the pedal con- nection of the control unit. The plug must lock in position. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 21: Mounting A Grasping Forceps (According To Option 3)
• Switch off the power switch on the back of the device (“O” position). • Plug the included power cable into the socket on the back of the device. • Plug the other end of the power cable into the power socket Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 22: Operation
Risk of injury The ERGO 300 - Morcellator - control unit has been designed for operation with the morcellation blades as a set. The user is solely responsible if different products are used with the set. Incompatibility may risk injury to patients. - Page 23 The values shown on the display are speed ranges, which can be called up by pedal. In operation the display does not show any current speed values. The values described can deviate from the actual values by a maximum of 10 %. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 24: Execution
Tissue may escape the gripper during the procedure and fall into the abdominal cavity. Causes: • The tissue is not gripped tightly enough. Remedy: • Grip the tissue at a different position • Press the jaws of the gripper together as tightly as possible. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 25 If the jaws of the gripper are not completely closed when it is pulled from the protective tube, the blade will be damaged (see pictures). Risk of injury! Damaged blades and cutting tubes must not be used. Cutting tubes must not be sharpened! Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 26: Option 1: Morcellation With Protective Tube
Make sure that the proximal opening of the seal unit is closed immediately with the thumb when there is no instrument in the Morcellator to prevent large quantities of gas from escaping. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 27: 6.2.2 Option 2: Morcellation With Trocar Cannula
Rotate the Trocar Cannula so the “CUT” position is visible from above. If the cutting tube is completely inserted, the cut-ting edge is now only partially covered by the Trocar Cannula. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 28 Grasp the tissue with the grasping forceps and pull it out through the trocar sleeve and sealing unit. Endoscope Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 29: Option 3: Myomektomy
Carefully pull the complete forceps out of the Morcellator and the patient. The retracted mouth must not touch the blade of the cutting tube. 6. Removing additional tissue If necessary, re-insert the myoma drill and repeat the above process. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 30: After Use
Pull the pedal device connection plug from the pedal connection of the control unit. 6. Preparing for preparation at the location of use Take the product to the preparation area immediately. Prepare the product as specified in this manual. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 31: Disassembly Of The Trocar Cannula
Trocar Cannula. 2. Remove the internal seal from the sealing cap holder. 3. Unscrew the valve body from the main body. 4. Remove the red O-ring from the valve body. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 32: Disassembly Of The Motor
Hold the cutting tube with the same hand and press it lightly against the gear unit until it releases itself from the holder and slides out smoothly. It can now be pulled out of the proxi- mal opening of the gear unit. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 33: Instrument Preparation
Risk of damage! Do not use incompatible preparation methods. The product may be damaged. Risk of damage to the ERGO 300 - Morcellator control unit • Wipe away dirt or dust with a soft cloth. • Use a moist cloth for stubborn dirt. -
Page 34: The Following Instruments Must Be Reprocessed
Aufbereitungs- Remove blood, secretions, tissue and bone residues with a disposable cloth immediately after the operation. Do not allow Vorbereitung to dry out! Dried residues cause corrosion. am Einsatzort Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 35 6. Rinse disassembled instruments and their attachments for 10 seconds from the outside with a water pressure gun (with a minimum pressure of 2.0 bar manufacturer HEGA Medical, REDF 6010 or 7060). Electronic motor ERGO 300 including cable, 3m. REF: 905-002 Wipe the electronic motor with a dampened disposable cloth and remove all visible dirt.
- Page 36 3 seconds. Oil introduced under pressure can escape from the pressure equalization opening of the gear unit. Then wipe the transmission unit with a damp cloth. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
- Page 37 The gripping pliers shown are not part of the “morcellator set”, but can be ordered as an option. The instructions required for reprocessing are described in detail in the instructions for use supplied with the respective gripper. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
-
Page 38: Maintenance And Repair
Roof shaped black seal (REF 5134nou) for cutting tubes Ø 20 mm, PU of 10 pieces. Maintenance, spray adapter for NouvaOil-Spray • Replace missing adapter by a new one Spray adapter (REF 905-030) for NouvaOil-Spray (REF 2127) for lubrication of the electronic motor 21 REF 905-030 Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 39: Replacing The Control Unit Fuse
The objective of this measure is to ensure that device defects and risks to patients, users or third parties are identified in time. The STI (Safety technical inspection) for the ERGO 300 - Morcellator shall be executed every 2 years by authorized experts. Results shall be documented. -
Page 40: Malfunction And Troubleshooting
Morcellator set with Trocar Cannula (option 2) If a fault cannot be rectified, please contact your supplier or an authorized service center. The addresses are provided on the last page of the operating instructions. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 41: Fault Messages On The Display
The pedal must be released for a short the device. period. If a fault cannot be rectified, please contact your supplier or an authorized service center. The addresses are provided on the last page of the operating instructions. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 42: Spare Parts List With Order Number
The national and local regulations for disposal of electronic waste must be observed. If they are not sterilized before disposal, please observe the national and local regulations for disposal of infectious waste. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 43: Appendix
Appendix Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... -
Page 44: Electromagnetic Compatibility (Emc)
Mains power quality should bet hat of a typical interruptions and voltage with 0, 45, 90, 135, 180, 225, with 0, 45, 90, 135, 180, 225, commercial or hospital environment. 270, 315 degree 270, 315 degree Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701... - Page 45 Product should b observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Product. over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m. Instructions for use, ERGO 300 - BOWA- IFU-12930- ERG0300-S0-EN-20210701...
- Page 46 Electromagnetic immunity against high-frequency wireless communication devices Test frequency Frequency Communication Modulation Maximum distance Test level band service Performance Pulse modulation 380 to 390 TETRA 400 18 Hz GMRS 460, 430 to 470 ± 5 kHz Hub FRS 460 1 kHz Sinus Pulse modulation 704 to 787 LTE Band 13, 17...
-
Page 47: Declaration Of Conformity
Nouvag Dental- und Medizintechnik GmbH Schulthaißstr. 15, 78462, Konstanz, DE SRN: DE-AR-000005643 erklären in alleiniger Verantwortung, dass das Medizinprodukt declare on our own responsibility that the medical device TCM 3000 BL Morcellator / (905-xxx – Morcellator ERGO 300) Klassifizierung Bezeichnung nach MDD GMDN... -
Page 49: Manufacturer And Service Points
Technical service For maintenance and repairs, contact the following address: BOWA MEDICAL BOWA-electronic GmbH & Co. KG Heinrich-Hertz-Strasse 4 – 10 72810 Gomaringen Deutschland Telefon: +49 7072-6002-0 Telefax: +49 7072-6002-33 E-Mail: service@bowa-medical.com oder im Internet unter: www.bowa-medical.com... - Page 52 D-78462 Konstanz Phone: +49 7531 1290-0 Fax.: +49 7531 1290-12 info-de@nouvag.com Vertrieb durch: BOWA MEDICAL BOWA electronic GmbH & Co. KG Heinrich - Hertz - Strasse 4 – 10 D-72810 Gomaringen • Germany Phone: +49 7072-6002-0 Fax: +49 7072-6002-33 info@bowa-medical.com • www.bowa-medical.com...
















Need help?
Do you have a question about the ERGO 300 and is the answer not in the manual?
Questions and answers