Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for mectron Piezosurgery II
- Page 1 English MANUAL OF USE AND MAINTENANCE 0476...
-
Page 2: Table Of Contents
MANUAL OF USE AND MAINTENANCE Summary 00.0 - INTRODUCTION ..........................3 00.1 - Foreword ............................3 00.2 - Description of the Device ......................3 00.3 - Intended Use ..........................4 00.4 - Safety requirements ........................4 01.0 - IDENTIFITION DATA ........................6 01.1 - Identification data ......................... -
Page 3: Introduction
The information and illustrations contained in this manual are up-dated to the date of publication indicated on page 32. MECTRON are committed to continuous up-dating of their products, which may entail changes to components of the equipment. If there are any discrepancies between the descriptions contained in this manual and your equipment, please contact your dealer or the MECTRON After-Sale service for explanations. -
Page 4: Intended Use
(anaesthetic mixtures, oxygen, etc.). 00.4 - Safety requirements Mectron will not accept any liability for direct or incidental personal injury or damage to property in the following cases: 1 If the equipment is used for purposes other than that for which it is intended. - Page 5 Follow the instructions provided under point “06.0” closely. DANGER: Use only original Mectron accessories and spare parts. DANGER: Check the condition of the device before treatment. Always make sure that there is no water under the apparatus. Before each treatment always check that the equipment is in proper working order and that the accessories are efficient.
-
Page 6: Identifition Data
An exact description of the model including the serial number of the equipment will make it easier for our After-Sale Service to respond quickly and efficiently to your queries. Always provide the above information whenever you contact a Mectron Service Centre. 01.2 - Data Plate of the Device Each device has its own data plate (Fig.1), on which its technical specifications and serial... -
Page 7: Testing
Special care must therefore be taken for both transport and storage. In order to avoid crushing, do not place cartons on top of one. All material shipped by MECTRON is checked at the time of shipment. The equipment is delivered properly protected and packaged. -
Page 8: List Of Materials Included In The Standard Supply
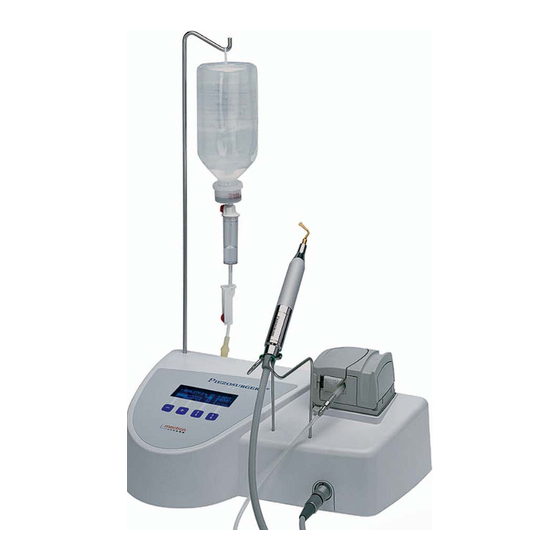
MANUAL OF USE AND MAINTENANCE 03.2 - List of Materials included in the Standard Supply 1 Casing of the device (Fig.3 - Ref.B). 2 Piezosurgery handpieces complete with cords (Fig.3 - Ref.E). 2 K4 torque wrenches K4 (Fig.3 - Ref.D). 1 Footswitch with cable and plug (Fig.3 - Ref.G). - Page 9 MANUAL OF USE AND MAINTENANCE Fig. 3 9 / 32 Piezosurgery // GB...
-
Page 10: Initial Installation
To ensure perfect operation of the equipment, it is installed by technical personnel author- ised by Mectron. The equipment will be installed in a suitable and handy place for it to be used, and is enabled by entering an activating password. - Page 11 MANUAL OF USE AND MAINTENANCE Fig. 4 11 / 32 Piezosurgery // GB...
- Page 12 MANUAL OF USE AND MAINTENANCE Fig. 5 12 / 32 Piezosurgery // GB...
-
Page 13: Use
MANUAL OF USE AND MAINTENANCE 05.0 - USE 05.1 - Controls This section illustrates the parts of the front panel (Fig.6) of the Piezosurgery unit, ena- bling the controls described in this manual to be located immediately. Description of the controls Ref. -
Page 14: Description Of The Display And Functions
MANUAL OF USE AND MAINTENANCE Fig. 6 05.3 - Description of the Display and Functions This point describes the three “screens” shown on the Piezosurgery display and their functions. With the Piezosurgery device, two different power modes can be used. These are ROOT and BONE. - Page 15 MANUAL OF USE AND MAINTENANCE Device in the BONE mode Fig. 8 - In the BONE mode, press the power-level key (Fig.6 - Ref.C) to set the following functions: QUALITY 1 QUALITY 2 QUALITY 3 SPECIAL To adjust the delivery rate of the pump press the + and – keys (Fig.6 - Rif.B). In the BONE mode, the speed can be set to between one and five.
-
Page 16: Safety Requirements During Use
MANUAL OF USE AND MAINTENANCE 05.4 - Safety Requirements during Use DANGER: Contraindications. Do not use the Piezosurgery on patients with pacemakers or other implantable electronic devices. This requirement also applies to the operator. DANGER: Breakage and wear of the inserts. High frequency oscillations and wear may, on rare occasions, lead to breakage of an insert. -
Page 17: Protection Systems And Alarms
ERR CKS Error signal. Firmware. Firmware checksum incorrect: - If the equipment is working properly, call as soon as possible. - If the equipment is not working correctly, stop using it and call the Mectron Service Centre immediately. 17 / 32... -
Page 18: Instructions For Use
MANUAL OF USE AND MAINTENANCE 05.6 - Instructions for Use 1 Open the air intake on the drip system. 2 Fit the required insert (Fig.10 - Ref.A) onto the Piezosurgery handpiece and lock it into place using one of the wrenches included in the supply. 3 To use the torque wrench correctly (Fig.10 - Ref.B), proceed as follows: - screw the insert onto the handpiece as far as it will go - hold the wrench and the casing of the handpiece very firmly... -
Page 19: Cleaning, Disinfection And Sterilisation
MANUAL OF USE AND MAINTENANCE 4 Always make sure that any threaded parts and their contact surfaces are perfectly clean. 5 If an insert becomes too worn, the device will stop working. 06.0 - CLEANING, DISINFECTION AND STERILISATION 06.1 - CLEAN function – Cleaning of the liquid circuit WARNING: Failure to carry out cleaning of the tubes will lead to crystallisation of salts that can seriously damage the equipment. -
Page 20: Sterilisation Procedure
MANUAL OF USE AND MAINTENANCE DANGER: The apparatus cannot be sterilised. After each treatment carry out the following operations: 1 Remove the insert from the scaler handpiece. 2 Clean and disinfect the surfaces of the casing, the cords and their connectors using a cloth moistened with a mild detergent or disinfectant solution with a neutral pH (pH 7). -
Page 21: Autoclave Sterilisation Of The Inserts
MANUAL OF USE AND MAINTENANCE WARNING: After completing the sterilisation cycle, allow the handpiece to dry completely before using it. NOTE: Water-based disinfecting solutions with a neutral pH are highly recommended. Some alcohol-based disinfecting solutions may be harmful and discolour or otherwise damage plastic materials. -
Page 22: Autoclave Sterilisation Of The Peristaltic Pump Tube
MANUAL OF USE AND MAINTENANCE 06.7 - Autoclave sterilisation of the peristaltic pump tube 1 Clean the peristaltic pump tube. 2 Disinfect with a mild disinfectant solution having a neutral pH and dry it thoroughly. 3 Seal the tube inside an individual disposable bag. 4 Autoclave sterilise the tube. -
Page 23: Regular Maintenance
3 Carry out cleaning, disinfection and sterilisation (see Section “06.0”) 07.2 - Power-supply Cable DANGER: Check regularly that the power cable is intact. If it is damaged, replace it with an original Mectron spare. 08.0 - REPLACEMENT OF THE FUSES DANGER : Switch off the apparatus. -
Page 24: Procedures And Precautions For Disposal
MANUAL OF USE AND MAINTENANCE 09.0 - PROCEDURES AND PRECAUTIONS FOR DISPOSAL The procedures and precautions to be observed for disposing of the apparatus are those indicated in the current regulations for any other electronic apparatus that is no longer in use. -
Page 25: Symbols
MANUAL OF USE AND MAINTENANCE Blunt inserts. Blunt inserts are used for separating the soft tissues, for example for detaching Schnei- der’s membrane or for lateralising nerves. In periodontology, these inserts are used to smooth the root surfaces. 11.0 - SYMBOLS N.B.: Please read carefully the instructions for use. -
Page 26: Troubleshooting
The footswitch will not work. Contact the nearest author- ised MECTRON Service Centre. A faint whistle can be heard The insert is not correctly Unscrew the insert and coming... - Page 27 The mes- vice sage ERR appears on the Lack of continuity of a lead display. Contact the nearest author- in the cord. ised MECTRON Service Centre. Handpiece failure. Contact the nearest author- ised MECTRON Service Centre. Malfunctioning of the tuning Contact the nearest author- circuit.
- Page 28 MANUAL OF USE AND MAINTENANCE PROBLEM POSSIBLE CAUSE SOLUTION The device is working prop- Too much pressure by the Check that the tube in the erly, but the pump is being impeller on the tube in the peristaltic pump has been forced.
-
Page 29: Technical Data
MANUAL OF USE AND MAINTENANCE 13.0 - TECHNICAL DATA Device in accordance with Directive 93/42/EEC: Class II a. Class according to EN 60601-1: Type B IP 20 (device) IP X8 (footswitch) Device for intermittent operation: 60” ON 30” OFF. Power-supply voltage: 230 VAC ±... - Page 30 MANUAL OF USE AND MAINTENANCE Alarms: The display on the front panel indicates error (see point “Problem-solving”). Operating conditions: From +10°C to +40°C. Relative humidity from 30% to 75%. Transport and storage conditions: From -10°C to +70°C. Relative humidity from 10% to 90%. Air pressure P: 500hPa/1060hPa Cord: It is recommended not to exceed 100 sterilisa-...
-
Page 31: Guarantee
In the event of failures due to accidents or improper use, or if the warranty has lapsed, repairs to MECTRON products will be charged on the basis of the actual cost of the materials and labour required for such repairs. - Page 32 Srl. Via loreto 15/A 16042 Carasco (Ge) Italy Tel. +39 0185 35361 Fax +39 0185 351374 www.mectron.com e-mail: mectron@mectron.com Reseller The information given in this manual is not binding and can be modified without prior notice.






Need help?
Do you have a question about the Piezosurgery II and is the answer not in the manual?
Questions and answers
What does Error 5 mean on the display?
Error 5 on the Mectron Piezosurgery II display indicates that the tuning scan has failed. Possible causes include:
- The insert is not correctly secured to the handpiece.
- The insert is worn, broken, or deformed.
- The electrical contacts of the cord are wet.
This answer is automatically generated