Table of Contents
Advertisement
Quick Links
PROFESSIONAL MEDICAL PRODUCTS
DOPPLER PORTATILE CON SCHERMO A COLORI
COLOUR SCREEN POCKET DOPPLER
ÉCRAN PORTATIF COULEUR DOPPLER
PANTALLA DE COLOR PORTÁTIL DOPPLER
MONITOR A CORES DETECTOR DOPPLER
DE BOLSO
FARBBILDSCHIRM-TASCHENDOPPLER
Manuale d'uso - User manual - Manuel de l'utilisateur
Guía de uso - Guia para utilização
Gebrauchs- und instandhaltungsanleitung
ATTENZIONE: Gli operatori devono leggere
e capire completamente questo manuale
prima di utilizzare il prodotto.
ATTENTION: The operators must carefully read
and completely understand the present manual
before using the product.
AVIS: Les opérateurs doivent lire et bien
comprendre ce manuel avant d'utiliser le produit.
ATENCIÓN: Los operadores tienen que leer y entender
completamente este manual antes de utilizar el producto.
ATENÇÃO: Os operadores devem ler e entender
completamente este manual antes de usar o produto.
ACHTUNG: Diese Anleitung muss vor dem Einsatz des
Produkts aufmerksam gelesen und vollständig verstanden werden.
29480 / Sonoline C
CONTEC MEDICAL SYSTEMS CO., LTD
No.112 Qinhuang West Street, Economic & Technical
Development Zone, Qinhuangdao, Hebei Province,
PEOPLE'S REPUBLIC OF CHINA
Made in China (P.R.C.)
Shanghai International Holding Corp. GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
0123
Advertisement
Table of Contents

Summary of Contents for Gima Sonoline C 29480
- Page 1 Gima S.p.A. Via Marconi, 1 - 20060 Gessate (MI) Italy gima@gimaitaly.com - export@gimaitaly.com www.gimaitaly.com PROFESSIONAL MEDICAL PRODUCTS DOPPLER PORTATILE CON SCHERMO A COLORI COLOUR SCREEN POCKET DOPPLER ÉCRAN PORTATIF COULEUR DOPPLER PANTALLA DE COLOR PORTÁTIL DOPPLER MONITOR A CORES DETECTOR DOPPLER...
- Page 2 ENGLISH Attention This user manual is written and compiled in accordance with the council directive MDD93/42/EEC for medical devices and harmonized standards. In case of modifications and software upgrades, the information contained in this document is subject to change without notice. The manufacturer makes no warranty of any kind with regard to this material, including, but not limited to the implied warranties of merchantability and fitness for a particular purpose.
-
Page 3: Chapter 1 Safety Guidance
ENGLISH Chapter 1 Safety Guidance This unit is internally powered equipment and the degree of shock protection is type CF applied part . Type CF protection means that these patient connections will comply with permitted leakage currents, dielectric strengths of IEC 60601-1. WARNING and CAUTION messages must be observed. - Page 4 ENGLISH electromagnetic interference, such as radio transmitters, mobile telephones, etc. Keep them far away. CAUTION: The user must check that the equipment does not have visible evidence of damage that may affect patient safety or monitoring capability before use. The recommended inspection interval is once per month or less. If damage is evident, replacement is recommended before use.
- Page 5 ENGLISH When disinfecting the machine: WARNING: Never try to sterilize the probe or equipment by low temperature steam or other methods. Refer to accompanying documents. Chapter 2 INTRODUCTION 2.1 Overview Pocket Fetal Doppler is a hand-held obstetrical unit, which can be used in hospital, clinic and home for daily self-check by pregnant woman.
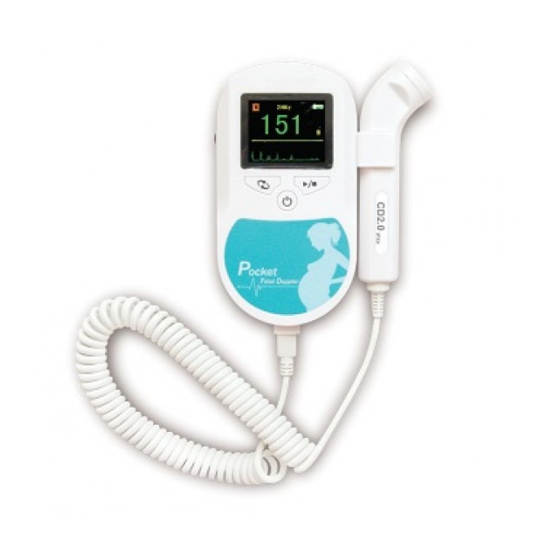
- Page 6 ENGLISH Chapter 3 Outlook and Configuration Mode Button Start/Stop Button Power Button Probe Fig. 3-1 Front Panel Speaker Battery Compartment Fig. 3-2 Rear Panel...
-
Page 7: Push Button
ENGLISH Headphone Headphone Socket Fig. 3-3 Top Panel 3.1 Display The LCD display is as follows: Probe Nominal Frequency Battery Status Work Mode Indicator FBR Value FHR Bar Graph FHR Heartbeat Waveform Fig. 3-4 LCD Display 3.2 Push Button There are three push button (Power, Mode, and Start/Stop) and a volume control button on Pocket Fetal Doppler. -
Page 8: Mode Button
ENGLISH 3.2.2 Mode Button Function: Mode selection, press once to enter next working mode under working status. For the Fetal Doppler has memory function, when turning on the machine, it will enter the mode selected before last power off automatically after self testing. -
Page 9: Chapter 4 General Operation
ENGLISH Headphone Socket Signal Interface: Fig. 3-5 Headphone Socket for Audio Output Headphone socket showed as Fig.3-5, the definition of pins showed as below: Definition Signal Signal Signal Signal Chapter 4 General Operation 4.1 FHR Inspection 1. Power on by pressing the Power button. The LCD display is as Fig.3-4. 2. -
Page 10: Mode Selection
ENGLISH 3. If pregnant woman adopts horizontal position and the fetus position is normal, put the probe on the position of lower navel midline to get the clearest FHR sound. 4. Do not measure FHR unless audible fetal sound has been heard. 5. - Page 11 ENGLISH 4.3.2 Replacing Probe There has been a probe connected to device while packaged by the manufacturer. If users need to replace it with another probe, power off the device at first, then take out the probe from the parking of device. And then pull out the plug of the probe from its socket.
-
Page 12: Battery Status Indicator
ENGLISH 4.4 FHR Alarm The normal fetal heart rate range is 120 BPM-160 BPM, LCD displays the fetal heart rate numerical values as green; when the fetal heart rate is too fast or too slow, beyond the normal range, the fetal heart rate numerical values red alarm to remind pregnant women to go to hospital for further checks to ensure the fetal safety. -
Page 13: Replacing Battery
ENGLISH 4.6 Replacing Battery 1. The rear panel is upturned. First open the battery compartment, then take out the battery from the battery compartment (see Fig.4-2). Fig. 4-2 Replacing Battery 2. Put two AA size batteries into the battery compartment (as for the direction of battery, please refer to the instruction inside the battery compartment), at last close the battery compartment. -
Page 14: Key Of Symbols
ENGLISH Chapter 5 KEY OF SYMBOLS SYMBOL DESCRIPTION TYPE CF REFER TO INSTRUCTION MANUAL/BOOKLET HEADPHONE SOCKET VOLUME ADJUST WEEE (2002/96/EC) EUROPEAN REPRESENTATIVE SERIAL NUMBER Chapter 6 PRODUCT SPECIFICATION Product Name: Pocket Fetal Doppler Safety: Complies with: IEC 60601-1:2005 Classification: Anti-electroshock Type: Internally powered equipment. Anti-electroshock Degree: Type CF applied part Harmful Liquid Proof Degree: Main unit: Degrees of protection provided by enclosure: IPXO. -
Page 15: Chapter 7 Maintenance
ENGLISH Weight: About 245g (including batteries) Environment Working: Temperature: +5°C~+40°C Humidity: <80% Atmospheric Pressure: 70 kPa~106 kPa Transport and Storage: Temperature: -10°C~+55°C Humidity: ≤93% Atmospheric Pressure: 50 kPa~106 kPa Display: 1.77”262K TFT display FHR Performance FHR Measuring Range: 50-240BPM (BPM: beat per minute) Resolution: 1BPM Accuracy: +-2BPM Power Consumption: <1 W... - Page 16 ENGLISH The user must check that the equipment does not have visible evidence of damage that may affect patient safety or Pocket Fetal Doppler capability before me. The recommended inspection interval is once per month or less. If damage is evident, replacement is recommended before use. The equipment should undergo periodic safety testing to insure proper patient isolation from leakage currents.
- Page 17 ENGLISH 2. After cleaning, sterilization and disinfecting, users must inspect whether have any obvious damage which may affect the patient safety and instrument performance. WARNING: Never try to sterilize the probe or equipment by low temperature. Chapter 8 SOLUTIONS FOR POSSIBLE PROBLEMS If it appears following problems when you use the device, please solve them as below: Problems...
- Page 18 ENGLISH Appendix 1 Essentiality of Fetal Domestic Monitor Modem medicine think that: FHR is an important gist to identify fetal health, by recording FHR changes can observe fetal hypoxia, fetal distress and the umbilical cord around the neck, and other symptoms. Fetal domestic monitor test FHR rate changes by listening to fetal heart sound mainly.
- Page 19 ENGLISH Appendix 2 Overall Sensitivity...
- Page 20 Congratulations for purchasing a GIMA product. This product meets high qualitative standards both as regards the material and the production. The warranty is valid for 12 months from the date of supply of GIMA. During the period of validity of the warranty, GIMA will repair and/or replace free of charge all the defected parts due to production reasons.














Need help?
Do you have a question about the Sonoline C 29480 and is the answer not in the manual?
Questions and answers