Gima SP-10 User Manual
Pocket spirometer with bluetooth
Hide thumbs
Also See for SP-10:
- User manual (160 pages) ,
- User manual (155 pages) ,
- User manual (52 pages)
Table of Contents
Advertisement
Quick Links
PROFESSIONAL MEDICAL PRODUCTS
SP-10 POCKET SPIROMETER
WITH BLUETOOTH
User manual
ATTENTION: The operators must carefully read and completely
understand the present manual before using the product.
33535 / SP10W
CONTEC MEDICAL SYSTEMS CO., LTD
ADD: No 112 Qinhuang West Street, Economic & Technical Development Zone,
Qinhuangdao, Hebei Province, 066004, PEOPLE'S REPUBLIC OF CHINA
Made in China
Shanghai International Holding Corp. GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Importer: Gima S.p.A. - Via Marconi, 1
20060 Gessate (MI) Italy
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
0168
0123
Advertisement
Table of Contents

Summary of Contents for Gima SP-10
- Page 1 ADD: No 112 Qinhuang West Street, Economic & Technical Development Zone, 0123 Qinhuangdao, Hebei Province, 066004, PEOPLE’S REPUBLIC OF CHINA Made in China Shanghai International Holding Corp. GmbH (Europe) Eiffestrasse 80, 20537 Hamburg, Germany Importer: Gima S.p.A. - Via Marconi, 1 20060 Gessate (MI) Italy...
- Page 2 ENGLISH Instructions to User Dear users, thank you very much for purchasing the SPIROMETER. Please read the User Manual carefully before using this product. The User Manual which describes the operating procedures should be followed strictly. Failure to follow the User Manual may cause measuring abnormality, equipment damage and human injury.
-
Page 3: Table Of Contents
ENGLISH Contents Chapter 1 Safety ..............................4 1.1 Instructions for safe operations ....................4 1.2 Warning ............................5 1.3 Attention ............................5 EMC declaration: ..............................6 1.4 Contraindication .......................... 6 1.4.1 Absolute contraindication ....................6 1.4.2 Relative contraindication ....................7 Chapter 2 Overview ............................ -
Page 4: Chapter 1 Safety
ENGLISH 6.1.6 Charge ..........................23 6.1.7 Upload data ........................23 6.2 Attention ............................ 24 Chapter 7 Maintenance,Transportation and Storage .................. 24 7.1 Cleaning and disinfection ......................24 7.2 Maintenance ..........................25 7.3 Transportation and storage ....................... 25 Chapter 8 Troubleshooting ..........................26 Chapter 9 Key of Symbols .......................... -
Page 5: Warning
ENGLISH 1.2 Warning Please don’t measure this device with functional tester for the device’s related information. Explosive hazard—DO NOT use the SPIROMETER in the environment with tinder such as anesthetic. Please check the packing before use to make sure the device and accessories are totally in accordance with the packing list, or else the device may have the possibility of working abnormally. -
Page 6: Emc Declaration
ENGLISH When data can’t be displayed at all times or other cases happened during testing, press “repeated measure” key to remeasure, or power off to restart. The device has normal life for three years since the first electrified use. ... -
Page 7: Relative Contraindication
ENGLISH 1.4.2 Relative contraindication • Heart rate >120 beats/min; • The one with pneumothorax or giant pulmonary bulla and not plan for surgical treatment; • The one with pregnancy; • The one with tympanic membrane perforation (need to block the ear canal of affected side before taking meas- urement);... -
Page 8: Features
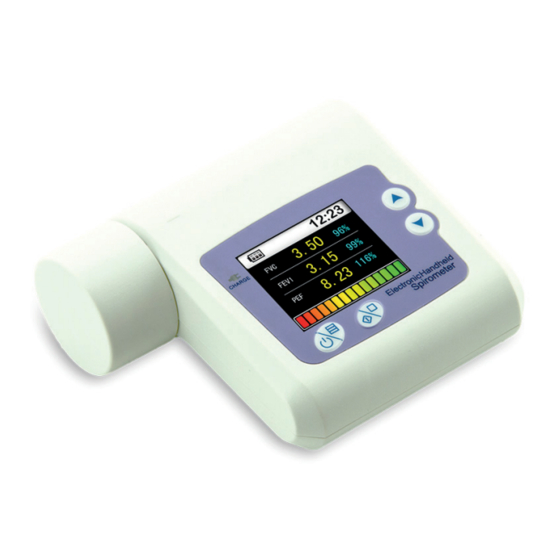
ENGLISH 2.1 Features 1) Ultra-thin design, concise and fashion. 2) Small in volume, light in weight and convenient in carrying. 3) Low power consumption. 4) TFT display. 5) Reflect lung function by measuring FVC, FEV1, PEF etc. 6) Take the function of wireless transmission. 2.2 Major applications and scope The SPIROMETER is a hand-held equipment for examining lung function. -
Page 9: Chapter 3 Principle
ENGLISH Chapter 3 PRINCIPLE Firstly, testee deep inspires, then seals the lips around the mouthpiece and blasts all air out as forcefully as possi- ble, the exhalant gas transforms to rotary airflow by turbine, then makes the blade rotate. The reception part of the infrared pair diodes (one is for infrared emission, the other is reception) towards to the blade is used for receiving the infrared ray, when the blade rotates, the received ray strength of the reception diode will be different as the difference of the blade angle, so form the various signal of same proportion in reception diode, which forms ac-... -
Page 10: Main Parameters
ENGLISH • Automatic power off when there is no operation in one minute. • Rechargeable lithium battery and with charging tips. • Battery power display. 4.2 Main parameters Volume Range: 0 L ~ 10 L Flow range: 0 L/s~16 L/s Volume accuracy: ±3% or 0.05L (whichever is greater) Flow accuracy: ±5 % or 0,2 L/s (whichever is greater) Working current: 60mA... -
Page 11: Chapter 5 Installation
ENGLISH Chapter 5 INSTALLATION 5.1 View of the front panel Turbine Charging indicator Up key Down key Power on, menu, confirm key Repeated measure: press it when you want (notice: Power off was controlled to next Measure (only need repeat measure by main menu) to press this key for a long time, other times please don’t press it... -
Page 12: Installation
ENGLISH 2) Insert the disposable mouthpiece into the turbine port. 5.3 Accessories 1) An User Manual 2) An USB data line 3) Disposable mouthpiece 4) A power adapter (optional) 5) A CD (PC software) 6) A nose clip (optional) Other type adapter should meet the following conditions: output voltage:DC 5V; output current ≥500mA, the power adapter must meet the requirements of EN60601 related standards and have the CE mark Chapter 6... -
Page 13: Measurement
ENGLISH 6.1.2 Measurement 1) Long press “Power On” key to turn on the power after installed. 2) The device is in selective interface after turn on as Figure 6-1, press “Up”, “Down” key to adjust, select “No” to “Testing” interface as Figure 6-2. Figure 6-1 Figure 6-2 3) Then breath in fully and seal the lips around the mouthpiece and blast all air out as forcefully as possible in best... - Page 14 ENGLISH Force Forced Peak Figure 6-3. Stat (Note: Status indicator bar indicates the measured state, displays the testee condition by the ratio of measured value and the predicted value. I.e. Compared the measured value with the reference value in same situation, when the value is lower than 50% indicates that should be noticed and hospitalized in time;...
-
Page 15: Menu Operations
ENGLISH Figure 6-4 Flow rate-volume chart Figure 6-5 Volume-time chart 6.1.4 Menu operations When testing, press “Menu” to its main-menu as Figure 6-6, then user can browse other parameters, and control setting, patient information, real time setting, power off etc. can be operated, the detail methods are as following: Figure 6-6. - Page 16 ENGLISH a. OTHER PARAMETERS Press “Other Par” in main menu to its sub-menu as Figure 6-7 which displays other parameters except for the three parameters in main menu (see following for details), press confirm key to return to the main menu (press “Up”, “Down”...
- Page 17 ENGLISH Figure 6-8. Figure 6-9 Figure 6-10 2) Review Information Select “Review Fun” to its sub-menu. If the previous display state is “OFF”, then press the confirm key to open the function (Note: the review function only can be opened when the case numbers are more than one), then the interface will jump to Number interface as shown in Figure 6-11.
- Page 18 ENGLISH Attention: Open the review function can browse all measured data, the path is:Enter the main menu as Figure 6-6, select “Patient Info”, press the confirm key to enter its sub-menu as Figure 6-20. Select “Number”, then press the confirm key to case number interface as Figure 6-11 (The function only can be selected in case of opening the review function), then use “Up”...
- Page 19 ENGLISH Figure 6-14 Figure 6-15 5) Wireless Transmission When select “Wireless”, if the device has built-in wireless module, press the confirm key to achieve turning on/off the wireless module, when it is ON, it can transmit data. 6) Scaler Operation(Calibration) Select “Scaler (Calibration)”...
- Page 20 ENGLISH Figure 6-16 Figure 6-17 Figure 6-18 Figure 6-19 7) Exit Select “Exit” to exit “Control Setting” interface and return to the main menu. c. PATIENT INFORMATION Press “Patient Info” to its sub-menu as Figure 6-20 (Note: Figure 6-1 is selective interface, and “Yes” indicates that you can edit patient information). Figure 6-20...
- Page 21 ENGLISH 1) Number “Number” is the current patient data.if you are the twelfth testee,the “Number” will display 12. Attention: If the review function is open,press the confirm key to case number interface to review patient’s infor- mation, refer to “Review Information” for the detailed operations. 2) Gender Settings Press “Up”, “Down”...
-
Page 22: Repeated Measure
ENGLISH Figure 6-21 Figure 6-22 5) Exit Press “Up”, “Down” key in “Personal Info” interface to “Exit” to exit “Patient Info” interface and return to the main menu. d. POWER OFF Press “Power Off” to turn off the device. Attention: the device will Automatic power off when there is no operation in one minute. e. -
Page 23: Charge
ENGLISH Figure 6-23 6.1.6 Charge There are two kinds of charging methods: 1) Connect the device with computer by data line – then the device should be under charging state. 2) Connect the device with power supply by power adapter, then the device should be under charging state. DO NOT use the device when it is under charging state. -
Page 24: Attention
ENGLISH 1) Connect the device with computer by data line, double press the icon to open the PC software procedure. 2) Press the corresponding key to achieve upload data, delete case, print information, background, select lan- guage, switch PDF format, set the testee information etc. 3) Press “Exit”... -
Page 25: Maintenance
ENGLISH 7.2 Maintenance 1) Please clean and disinfect the device before using according to the User Manual (7.1). 2) Please recharge the battery when the screen shows low-power (the battery power is 3) Recharge the battery soon after the over-discharge. The device should be recharged every six months when it is not regular used. -
Page 26: Chapter 8 Troubleshooting
ENGLISH Chapter 8 TROUBLESHOOTING Trouble Possible Reason Solution The device can't finish The start speed is too low, Remeasure according to the user manual. measurement for a long the device does not measure. time, and the data can't The malfunction of the device. Press “Repeated Measure”... - Page 27 ENGLISH The device can not be used The battery is not full charged. Please recharge the battery. for full time after charge The battery is broken. Please contact the local service center. The battery can not be The battery is broken. Please contact the local service center.
-
Page 28: Chapter 9 Key Of Symbols
ENGLISH Chapter 9 KEY OF SYMBOLS Symbol Meanings Please read instructions carefully IP22 International Protection Read instructions carefully WEEE Type BF Applied part Full-power Low-power Error Measured value goes beyond the limits Authorized representative in the European community Keep away from sunlight... - Page 29 ENGLISH Status indicator bar 106kPa Atmospheric pressure limitation 50kPa Humidity limitation +55% Temperature limitation -40% Fragile, handle with care Keep in a cool, dry place This way up Manufacturer Date of manufacture. Serial number...
-
Page 30: Chapter 10 Parameter Introduction
ENGLISH Chapter 10 PARAMETER INTRODUCTION Measured parameters Parameter Description Unit Forced vital capacity FEV1 Forced expired volume in one second Peak expiratory flow FEV1% FEV1/FVC×100 FEF25 25% flow of the FVC FEF2575 Average flow between 25% and 75% of the FVC FEF75 75% flow of the FVC... -
Page 31: Appendix
ENGLISH Appendix Guidance and manufacturer’s declaration – electromagnetic emissions for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration – electromagnetic emission The Spirometer is intended for use in the electromagnetic environment specified below. The customer of the user of the Spirometer should assure that it is used in such and environment. Emission test Compliance Electromagnetic environment –... - Page 32 ENGLISH Guidance and manufacturer’s declaration – electromagnetic immunity – for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration – electromagnetic immunity The Spirometer is intended for use in the electromagnetic environment specified below. The customer or the user of Spirometer should assure that it is used in such an environment. Immunity test IEC 60601 Compliance...
- Page 33 ENGLISH Guidance and manufacturer’s declaration – electromagnetic immunity – for EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING Guidance and manufacturer’s declaration – electromagnetic immunity The Spirometer is intended for use in the electromagnetic environment specified below. The customer or the user of Spirometer should assure that it is used in such an environment.
- Page 34 ENGLISH Field strengths from fixed RF transmitters, as determi- ned by an electromagnetic site survey, should be less than the compliance level in each frequency range. Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations.
- Page 35 ENGLISH Recommended separation distances between portable and mobile RF communications equipment and the EQUIPMENT or SYSTEM – for EQUIPMENT or SYSTEM that are not LIFE-SUPPORTING Recommended separation distances between portable and mobile RF communications equipment and the Spirometer The Spirometer is intended for use in an electromagnetic environment in which radiated RF disturbances are con- trolled.











Need help?
Do you have a question about the SP-10 and is the answer not in the manual?
Questions and answers