Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Acteon ImplantCenter M+
- Page 1 User Manual ImplantCenter M+...
-
Page 3: Table Of Contents
Contents 1 Documentation 1.1 Associated documentation 1.2 Electronic documentation 2 Required information 2.1 Indication for use 2.2 Operating principle 2.3 Date of first inclusion of EC marking 2.4 Latest document update 2.5 Repairing or modifying the medical device 2.6 Warranty 2.7 Accessory usage conditions 3 Removal from packaging, installation, connections 3.1 Removing the medical device from its packaging... - Page 4 6.2 Switching on the medical device 6.3 Selecting the operating mode 6.3.1 Common operations using the footswitch 6.4 Connecting and disconnecting M+ accessories during use 6.5 Switching off the medical device 7 Configuring the medical device 7.1 Adjusting the medical device volume 7.2 Adjusting the screen brightness 7.3 Adjusting the lighting timer 7.4 Recovering the medical device's factory settings...
- Page 5 15 Glossary...
- Page 7 Foreword The SATELEC ® medical device that you are about use is designed for professional use only. It is therefore a key tool with which you will provide treatment within the context of your work. These medical devices are designed to be used exclusively within a hospital or private clinic operating theatre.
-
Page 9: Documentation
1 Documentation This document contains the following information: indications for use medical device description installation of the medical device medical device use preparation prior to cleaning and disinfecting the medical device monitoring and general maintenance of the medical device maintenance to be performed by the user. 1.1 Associated documentation This document must be used in association with the following documents: Document title... - Page 10 writing. The electronic user instructions are available in PDF format (Portable Document Format) and you will need to have a PDF file read software installed to read the instructions. The device user instructions can be consulted at the following address: www.satelec.com/documents It is important for you to have read and understood the content of the user instructions relating to the use of your device and its accessories prior to use.
-
Page 11: Required Information
2 Required information 2.1 Indication for use ImplantCenter ™ M+ is a surgical device that combines an M+ surgical or ultrasonic piezoelectric handpiece and a rotary surgical motor. The Piezotome ® M+ piezo-ultrasonic surgical handpiece can be used in combination with M+ tips for cutting into bone, metal and bone substitutes. -
Page 12: Warranty
SATELEC ® at the request of technical personnel working for the network of dealers approved by SATELEC ® , provide all information required to repair the faulty parts on which they may perform repairs. 2.6 Warranty The user may not remove any of the screws shown on this view, as this would invalidate the medical device's warranty. -
Page 13: Removal From Packaging, Installation, Connections
3 Removal from packaging, installation, connections 3.1 Removing the medical device from its packaging When you receive your medical device, check for any damage that may have occurred during transportation. If you have received this medical device by mistake, please contact the supplier to arrange for it to be collected. -
Page 14: Fixing The Medical Device To A Non-Removable Support
Do not plug the medical device into an extension lead and do not put the mains cord in a cable cover or cable tray. A different voltage would cause damage to the medical device and could injure the patient and/or user. Any variation in the electrical network voltage or electromagnetic field that is non-compliant with the limits in force, could interfere with the medical device's operation. -
Page 15: Medical Device Description
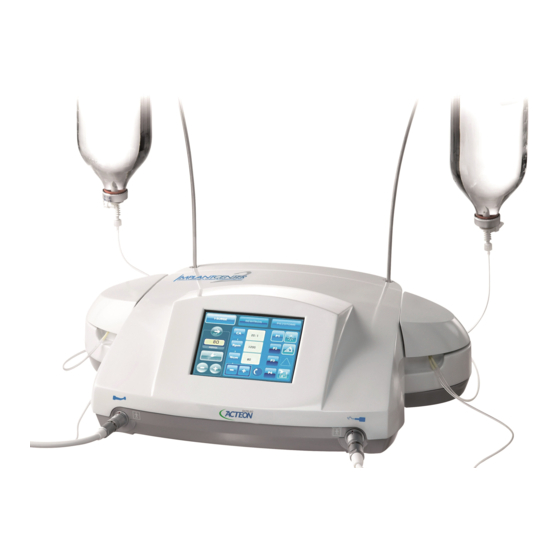
4 Medical device description 4.1 Control unit The control unit is compatible with: 1. The I-surge LED rotary technology via an I-surge LED surgical micromotor. 2. Newtron ® ultrasonic piezoelectric technology via the surgical Piezotome® M+ handpiece or via the Newtron®... -
Page 16: User Interface
5 User interface Pictograms Meaning shared by the three modes Start/stop purge Displays the flow in ml/min Irrigation ON Irrigation OFF Adjusts the irrigation flow rate. Handpiece lighting on Handpiece lighting off Progressive footswitch Smooth footswitch Warning. The type of warning is specified by a pictogram at the centre of the triangle. Saves any changes 5.1 I-surge mode Before using the I-Surge mode, read the setting recommendations specified by the contra-angle or... -
Page 17: Piezotome® M+ Mode
Pictograms Meaning for the I-Surge mode Select programme P4 - Screwing Clockwise rotation Anti-clockwise rotation I-Surge mode programme settings Programme Contra-angle Speed Torque Irrigation Function ratio at end of tool at end of tool 20:1 1200 rpm 80 N.cm 80 ml/min Implant site marking 20:1 800 rpm... -
Page 18: Newtron® Mode
Programme Fine setting level Power value Frequency modulation 60 Hz Programme Fine setting level Power value Frequency modulation 60 Hz Programme Fine setting level Power value Frequency modulation 30 Hz 5.3 Newtron mode ® Pictograms Meaning Power for the Newtron mode Soft Mode Medium Mode Medium... -
Page 19: Medical Device Use
6 Medical device use 6.1 Preparing the medical device 6.1.1 Connecting the medical device to the electrical network Set the ON/OFF switch to O position (off). Connect the power cord to the device's mains connector. Connect the mains power cord to the mains socket equipped with an earthing pin. If necessary, connect the potential equalisation cable to the medical device's potential equalisation terminal marked with 6.1.2 Footswitch... -
Page 20: Purging The Medical Device
6.1.8 Purging the medical device The medical device must be purged before and after use. 1. Immerse the irrigation line perforator in a container with distilled water. 2. Press to rinse the irrigation line and the I-Surge LED micromotor or handpiece. 3. - Page 21 6.4 Connecting and disconnecting M+ accessories during use Do not connect/disconnect the cords when the medical device is switched ON and the footswitch is pressed. Do not tighten or loosen the contra-angles when the I-Surge LED micromotor is activated. Do not tighten or loosen the tips when the handpiece is activated. 6.5 Switching off the medical device To avoid damaging the I-Surge LED micromotor, it must be rinsed with sterile water less than 30 minutes after use.
- Page 22 7. Disconnect the I-Surge LED micromotor cord and the medical device's handpiece cord. 8. Remove the irrigation line clips and dispose of them in a special container for soiled medical equipment. 9. Disconnect the irrigation line from the I-Surge LED micromotor or handpiece and dispose of it in a special container for soiled medical equipment.
- Page 23 7 Configuring the medical device In addition to normal use of the medical device, the following parameters can be configured: medical device volume screen brightness lighting timer factory settings 7.1 Adjusting the medical device volume 1. Switch on the medical device. 2.
- Page 24 7.4.1 I-Surge mode factory settings Programme Contra-angle ratio Speed at end of tool Torque at end of tool Irrigation 20:1 1200 rpm 80 N.cm 80 ml/min 20:1 800 rpm 80 N.cm 100 ml/min 20:1 15 rpm 20 N.cm 100 ml/min 20:1 30 rpm 20 N.cm...
- Page 25 8 Cleaning, disinfecting and sterilising The instructions relating to cleaning, disinfection and sterilisation protocols for accessories provided by SATELEC ® have been approved for each medical device and accessory. The applicable protocols are listed in the chapter Associated documentation page 7. They can be downloaded at the following address: www.satelec.com/documents In all cases, the local regulations in force relating to the cleaning, disinfection and sterilisation protocols for...
- Page 26 User Manual • ImplantCenter ™ M+ • J27251 • V1 • (13) • 09/2013 • NO25EN010A - Page...
- Page 27 9 Monitoring and general maintenance of the medical device Before and after use, check the medical device and its accessories entirely for any problems. This is necessary to detect any isolation fault or damage. If necessary, replace damaged parts. Check that the air inlets on the control unit are clean to prevent any overheating. The clips holding the irrigation lines may cause wear of the handpiece cords.
- Page 28 User Manual • ImplantCenter ™ M+ • J27251 • V1 • (13) • 09/2013 • NO25EN010A - Page...
- Page 29 10 Maintenance Maintenance of the medical device essentially involves preventive maintenance operations, covering the following aspects: checking of accessories; everyday cleaning, disinfection and sterilisation ; cleaning. 10.1 Touch-sensitive screen messages Use the touch-sensitive screen to configure the medical device. Depending on your actions, one or more of the following elements will be displayed.
- Page 30 Possible causes Solutions - Connect the I-Surge LED micromotor cord to the medical device. - Switch off the medical device (I/O switch to O). Faulty connection between the I-Surge LED micromotor cord and the medical device - Wait 5 seconds before switching it back on. - Switch the device on (I/O switch to I) and don't touch any of the buttons while it is starting up.
- Page 31 Possible causes Solutions chapter Associated Refer to the tip user manual indicated Angle of approach incorrect or inadequate pressure on the clinical site documentation page 7 Moisture on the I-Surge LED micromotor or handpiece Dry the electrical contacts cord connector 10.2.3 No spray or very little amount of spray Symptoms: when the device is in use, irrigation is not working and no spray comes out of the contra-angle or the tip...
- Page 32 10.3 Corrective maintenance In the event of faulty operation, the following corrective maintenance actions may be performed by the user. 10.3.1 Replacing the fuses The medical device is protected by two fuses in the mains connector. To replace the fuses, perform the following operations: stop the medical device (position O);...
- Page 33 11 Technical specifications of the medical device 11.1 Identification SATELEC ® Manufacturer ™ M+ Name of the medical device MPLANT ENTER 11.2 Control unit Width (in mm) 472.9 Height (in mm) 149.5 - 471.1 with bracket Depth (in mm) 339.9 Weight 5 kg without accessories Ingress protection rating: IPX0...
- Page 34 11.6 I-Surge LED micromotor Length (in mm) 93.1 Maximum diameter (in mm) 23.2 Weight (in g) 119 without cable Coupling as per ISO standard 3964 11.7 Irrigation ® ™ Nominal water output flow at the handpiece tip of the Piezotome M+ and the ImplantCenter 10 to 120 ml (in ml/min)
- Page 35 12 Regulations and standards 12.1 Official texts This medical device complies with the essential requirements of European Directive 93/42/EEC. This equipment is designed and developed in compliance with the Electrical Safety standard IEC60601-1 in force. It was designed and manufactured in accordance with an EN ISO 13485-certified quality assurance system. This documentation complies with European regulation No.207/2012.
- Page 36 : no ingress of protection rating claim against the penetration of solids : protected against the effects of continuous immersion in water 12.4 Manufacturer identification SATELEC A Company of ACTEON Group 17, avenue Gustave Eiffel BP 30216 33708 MERIGNAC cedex FRANCE Tel.
- Page 37 ACTEON INDIA e-mail: info@de.acteongroup.com B-94, GIDC Electronic Estate - Sector 25 – SPAIN GANDHINAGAR 382028 Gujarat - INDIA ACTEON MEDICO-DENTAL IBERICA, S.A.U. Tel. +91 79 2328 7473 Avda Principal n°11 H Fax. +91 79 2328 7480 Poligono Industrial Can Clapers e-mail: info@in.acteongroup.com...
- Page 38 ACTEON TAIWAN 14F-1, N°433, Jinping Rd. Jhonghe Dist., New Taipei City 23563 TAIWAN (R.O.C) Tel. + 886 926 704 505 e-mail: tina.chu@tw.acteongroup.com User Manual • ImplantCenter ™ M+ • J27251 • V1 • (13) • 09/2013 • NO25EN010A - Page...
- Page 39 When your medical device has reached the end of its service life, contact your nearest equipment dealer, or ACTEON GROUP head office or one of the company branches to find out how to proceed. The relevant contact details are given in chapter Branch addresses page 35.
- Page 40 User Manual • ImplantCenter ™ M+ • J27251 • V1 • (13) • 09/2013 • NO25EN010A - Page...
- Page 41 Index: – approved dealers user documentation fuses 13, 28, 30 water leakage 14 Index indexing points Manufacturer medical class approved dealers medical device attachment air inlets attachment system Cruise Control® risk of fall footswitch light function mains connector 13, 30 peristaltic pump settings Date...
- Page 42 Index: – approved dealers incorrect operation instructions for the entire range of M+ ultrasonic generators paper documentation Quick Clean Quick Reference Quick Start update user manual User manual User Manual • ImplantCenter ™ M+ • J27251 • V1 • (13) • 09/2013 • NO25EN010A - Page...
- Page 43 with the device, or instructions for use available through a website [European regulation No.207/2012] 15 Glossary professional users persons using the medical device in the course of their work and in the fra- accessory mework of a professional healthcare includes micromotors, cords, hand- activity [European regulation pieces, nozzles, LED rings, optical No.207/2012]...
- Page 44 Ref - J27251 • V1 • 09/2013 • NO25EN010A A Company ofACTEON Group• 71 av.GustaveEiffel • BP30216 • 33708 MERIGNAC cedex • France Tel.+33 (0)556 34 06 07 • Fax.+33 (0)556 34 92 92 E-mail:satelec@acteongroup.com • www.acteongroup.com...
















Need help?
Do you have a question about the ImplantCenter M+ and is the answer not in the manual?
Questions and answers