Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summarization of Contents
Indications for Use
Electrotherapy Indications
Lists uses for electrotherapy like muscle spasm relaxation, prevention of atrophy, etc.
Ultrasound Therapy Indications
Describes ultrasound therapy for deep heat, pain relief, muscle spasms, etc.
Section I: Introduction
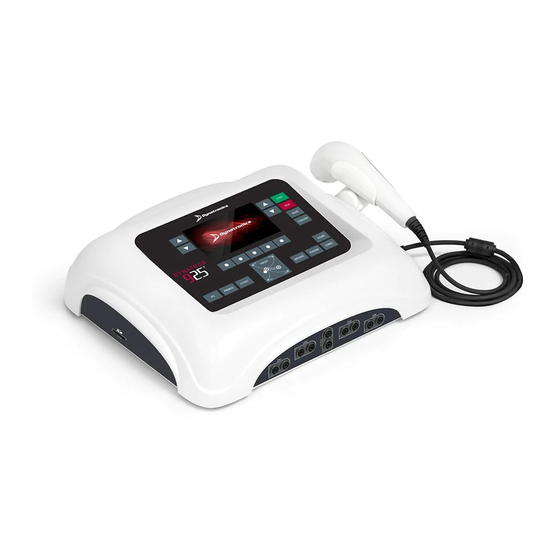
Dynatron 25 Series Overview
Provides a general introduction to the Dynatron 25 Series devices.
Installation and Features
Unpacking and Components
Instructions for unpacking the unit and listing standard components.
Dynatron® 25 Series Physical Features
Physical Feature Overview
Details the physical layout and main features of the device.
Section II: Operation and Treatment Instructions
Interferential / Premodulated Instructions
Setup and operation for Interferential and Premodulated treatments.
Biphasic / Russian Instructions
Setup and operation for Biphasic and Russian stimulation modes.
High Volt Instructions
Setup and operation for High Volt treatments.
Ultrasound Instructions
Setup and operation for Ultrasound therapy.
Ultrasound Modality Information
Soundhead Selection and Penetration
Guidance on choosing soundheads and understanding ultrasound penetration.
Ultrasound Problem Solving
Troubleshooting Common Ultrasound Issues
Addresses soundhead warming, coupling, and error messages.
Combination Therapy Instructions
Comboplus Feature and Setup
Details the Comboplus feature and how to perform combination treatments.
Section III: Contraindications, Warnings, and Precautions
Stimulation Therapy Contraindications
Lists conditions where stimulation therapies are contraindicated or require caution.
Ultrasound Therapy Contraindications
Lists conditions and areas where ultrasound is contraindicated or requires caution.
Section IV: Technical Information
Managing Device Defaults
How to set, save, and restore default treatment parameters.
Battery Operation
Battery Usage and Life
Instructions for using the battery and factors affecting its life.
General Specifications
Device Specifications and Environment
Technical specs, operating, and storage conditions for the device.
Basic Troubleshooting Techniques
Lead and Pad Testing
Procedures for testing leads and carbon pads for conductivity.
Electromagnetic Emissions and Immunity
Electromagnetic Emissions Guidance
Declarations on RF, harmonic emissions, and voltage fluctuations.
Electromagnetic Immunity Guidance
Guidance on electrostatic discharge, transients, surge, and voltage variations.
RF Communications Equipment Separation
Recommended distances for RF communication devices to avoid interference.
RF Separation Distance Table
Table showing recommended separation distances based on transmitter power.
Medical Device Reporting Requirements
Reporting Incidents and Serious Injury
Procedures for reporting device-related incidents and defining serious injury.
Installation and Features
Unpacking and Components
Instructions for unpacking the unit and listing standard components.
Dynatron® 25 Series Physical Features
Soundhead and Accessory Information
Lists available soundheads and optional accessories for the device.
Electrotherapy Usage Cautions
Carbon and Self-Adhesive Electrode Care
Guidelines for using, caring for, and maintaining electrodes.
Dynatron® 25 Series Physical Features
Control Panel Overview
Describes the physical layout and components of the device's control panel.
Control Panel Functions
Key Functions and Navigation
Details the function of keys like START, STOP, FUNCTION, ARROW keys, and MODALITY keys.
Treatment Windows and Focus
Explains the treatment windows and how to change the focus treatment.
Target Pad Operation
Describes the use of the Target Pad for precise treatment placement.
Electrotherapy Information and Usage Cautions
General Electrotherapy Warnings
Key safety warnings for all electrotherapy modalities.
Interferential / Premodulated Instructions
IFC/Premod Setup Basics
Basic steps for setting up Interferential or Premodulated treatments.
Default IFC/Premod Settings
Lists the default parameters for Interferential and Premodulated treatments.
Interferential and Premodulated Modality Information
Interferential Therapy Explained
Details how Interferential therapy works, including waveforms and electrode placement.
Biphasic / Russian Instructions
Choosing Treatment Modes
Explains the four treatment options for Biphasic and Russian stimulation.
Biphasic / Russian Modality Information
Russian Stimulation Details
Explains Russian stimulation, its wave, modes, and parameters.
Biphasic Stimulation Details
Explains Biphasic stimulation and its parameters.
High Volt Instructions
High Volt Electrode Setup
Describes electrode placement and setup for High Volt treatments.
High Volt Modality Information
High Volt Waveform and Settings
Explains the High Volt waveform and available settings like pulse rate and polarity.
Ultrasound Instructions
General Ultrasound Warnings
Critical safety warnings for using Ultrasound therapy.
Ultrasound Modality Information
Soundhead Selection
Guidance on choosing the correct soundhead based on treatment area size.
Ultrasound Problem Solving
Whirlpool Treatments and Soundhead Caution
Addresses soundhead overheating cautions during whirlpool treatments.
Warming a Cold Soundhead
Methods for warming a soundhead that is too cold to operate.
Combination Therapy Instructions
General Combination Therapy Warnings
Critical safety warnings for using combination therapy.
Simultaneous Treatments
Setting Up a Second Treatment
Steps to start a second or subsequent treatment.
Contraindications, Warnings, & Precautions (Stim)
General Stimulation Warnings
Additional warnings for stimulation, including thoracic applications.
Technical Information
Save New Defaults
Steps to change default treatment settings to custom preferences.
Battery Operation
Approved Battery Usage
Guidelines for using approved batteries and charging.
General Specifications
Dynatron 25 Series Specifications
Technical specs including power, dimensions, and weight.
Environmental Conditions: Transport and Storage
Specifies storage conditions for the device.
Basic Troubleshooting Techniques
Lead Testing Procedure
Step-by-step guide for testing leads using the console's function.
Electromagnetic Emissions and Immunity
Electromagnetic Emissions Guidance
Declarations on RF, harmonic emissions, and voltage fluctuations.
Medical Device Reporting Requirements
Definition of Serious Injury
Defines what constitutes a "serious injury" for reporting purposes.









Need help?
Do you have a question about the Dynatron 625 and is the answer not in the manual?
Questions and answers