EKO CORE Manual
- Quick start manual (68 pages) ,
- Manual (24 pages) ,
- User manual (23 pages)
Advertisement
- 1 Indications/Intended Purpose
- 2 Introduction
- 3 For Help and Assistance
- 4 Equipment Symbols
- 5 Cautions and Warnings
- 6 Contents and Compatible Operation
- 7 Installation to Existing Stethoscopes
- 8 CORE Use
- 9 Cleaning
- 10 Operating Conditions
- 11 CORE Modes and Corresponding LED States
- 12 Eko App
- 13 Electrical Emission and Device
- 14 Clinical Benefits
- 15 Network Security
- 16 Cybersecurity
- 17 Patient Privacy
- 18 Documents / Resources

Indications/Intended Purpose
The Eko CORE™ is an electronic stethoscope that enables amplification, filtering, and transmission of auscultation sound data (heart, lungs, bowel, arteries, and veins), whereby a clinician at one location on network can listen to the auscultation sounds of a patient on site or at a different location on the network. Eko CORE™ is intended for use on pediatric and adult patients. The Eko CORE™ is intended to be used by professional users in a clinical environment or by lay users in a nonclinical environment. The device is not intended for self-diagnosis.
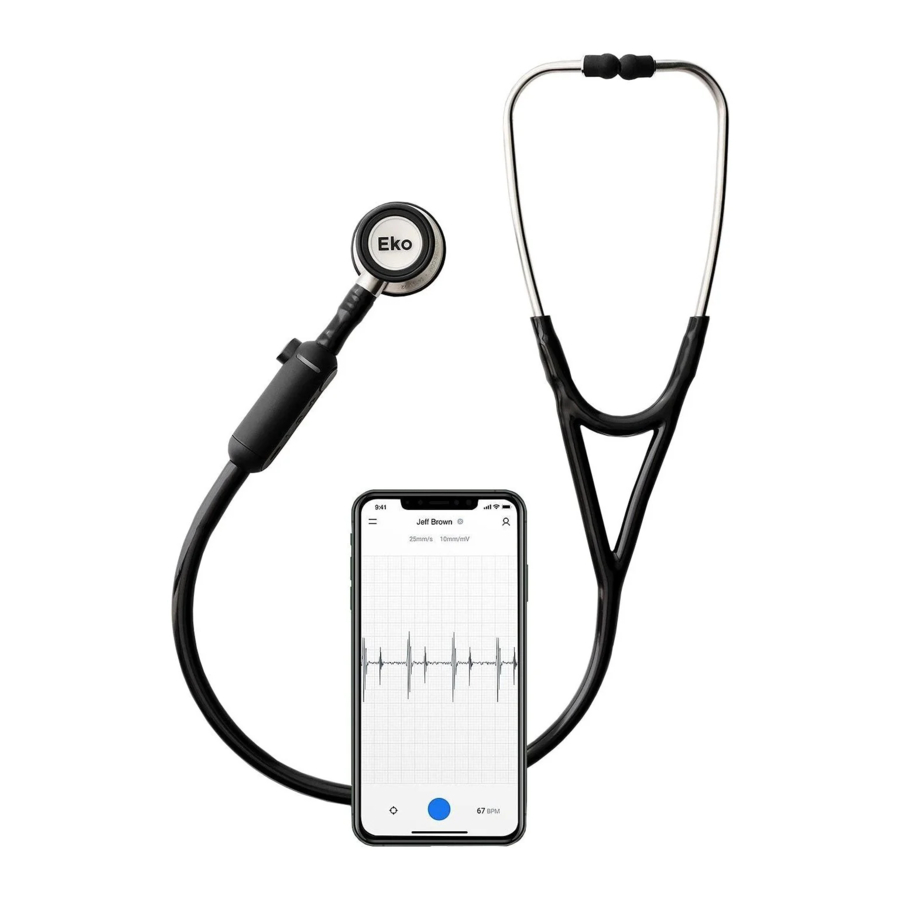
Figure 1
Fully assembled digital stethoscope and mobile app

Introduction
The Eko CORE™ (CORE™) is designed to support healthcare professionals in listening to sounds produced by the body, primarily lung, heart, and bowel sounds. CORE™ also enables regular users to record, store and share their body sounds with their physician. CORE™ includes a device that is attached to a stethoscope (Eko CORE™ Digital Attachment/CORE™ attachment) and an application, the Eko App. (Select features may require a paid subscription.)
CORE™ features sound amplification and audio transmission to a smartphone via Bluetooth that allows the user to open and playback sounds in a mobile application on compatible iOS and Android smartphones and tablets. The App provides the ability for clinicians to save sounds within selected Electronic Health Record (EHR) systems, share recordings with other clinicians, and annotate notes on recorded audio.
For Help and Assistance
Please contact Eko if you need assistance or any product related concerns.
For more information please visit: https://www.ekohealth.com/ifu
Direct Contact: support@ekohealth.com
Phone Support: 1.844.356.3384 This User Manual also applies to:
Eko CORE™ Digital Stethoscope
Eko CORE™ Digital Attachment 3M™ Littmann® CORE Digital Stethoscope
Equipment Symbols
 | Consult instructions for use Restrictions or Requirements in the UK |  | IP22 indicates protection against access to hazardous parts with a finger, solid objects ≥ 12.5 mm diameter, and vertically falling water drops when enclosure tilted up to 15 degrees. |
 | Consult Accompanying Documents | ||
 | CE Mark |  | Quantity |
 | Authorized Representative in the European Union |  | MR Unsafe |
 | WEEE Directive 2012/19/EU: Separate collection facilities for recovery and recycling |  | Catalogue number |
 | Medical device | ||
 | Non-ionizing electromagnetic radiation |  | Unique device identifier |
 | Model number | ||
 | Certified compliance with the Federal Communications Commission (FCC) rules |  | Serial number |
 | Caution |  | Type BF Applied Part |
 | Wireless Bluetooth communication |  | (IEC 60601-1) Transport and storage between uses -40°C to +55°C, at an atmospheric pressure range of 700 hPa to 1060 hPa |
 | Manufacturer | ||
 | Date of manufacture |  | Transport and storage between uses relative humidity range of 15% to 93%, at an atmospheric pressure range of 700 hPa to 1060 hPa |
 | Importer | ||
Cautions and Warnings
To reduce the risk of device interference, keep CORE™ at least 1 meter away from all RF emitters including Wifi routers and radios.
Follow all cleaning and disinfecting instructions included in this manual. Establish and follow a cleaning and disinfecting schedule.
To reduce the risks associated with inaccurate data acquisition store and operate this stethoscope only as instructed in this manual. It is highly recommended that the battery be recharged within thirty minutes of the LED indicator turning orange. Recharge the battery using only the provided USB power cord with a UL-certified USB wall charger (not provided).
DO NOT immerse the stethoscope in a liquid or subject it to any sterilization processes other than those described in this manual.
To reduce the risks associated with very strong electromagnetic fields avoid using the stethoscope near strong radio frequency (RF) signals or portable and/or mobile RF devices and/or specific RF emitters that are known sources of electromagnetic disturbance such as diathermy, electrocautery, RFID, security systems (e.g.,
electromagnetic anti-theft systems, and metal detectors). Interference from hidden RF emitters like RFID might cause packet loss and this will be visible as a "Poor Bluetooth Signal" message on the mobile application. Move away from the hidden RF emitter if this happens.
If sudden or unexpected sounds are heard, move away from any radio transmitting antennas. Using accessories, transducers, and cables not produced by Eko Devices, Inc. may result in increased RF emissions or decreased immunity of the CORE™.
Please read, understand, and follow all safety information contained in these instructions prior to using the CORE™. It is recommended that these instructions be retained for future reference.
To reduce the risk of thermal hazard by excessive voltage, always use a CSA/UL/ CE marked USB-IF charger from a trusted manufacturer to charge the Eko CORE™. CORE™ contains a Bluetooth wireless data link. The maximum radio frequency field strength generated by the stethoscope is below three volts per meter, a level that is considered safe to use with other medical devices. However, audio, video, and other similar equipment may cause electromagnetic interference. If such devices are encountered and cause interference, immediately move CORE™ away from that device and/or turn the Bluetooth feature OFF.
Consult with your physicians when using the Eko device.
To ensure high quality sounds location and position of CORE™ placement should be taken into consideration when auscultating.
To improve Bluetooth connection, reduce the distance and/or allow a line of sight between Eko device and mobile device. The Bluetooth range will be reduced when objects (walls, furniture, people, etc) are between the Eko device and a paired mobile device.
To reduce risk of asphyxiation and strangulation, ensure that all components are properly attached and stored. Keep away from children.
Allergic Reaction: Components of Eko CORE™ Digital Stethoscope are not known to cause allergic reactions. DO NOT continue to use if you have an allergic reaction to the device materials or if your skin appears irritated or inflamed after use. Check with a healthcare professional before restarting use.
Do not use Eko CORE™ Digital Stethoscope in an oxygen-rich environment!
Contents and Compatible Operation
CORE™ device includes (1) Eko CORE™ Digital Attachment, (2) tubing adapters, and (1) micro USB cable.
CORE™ Digital Stethoscope and 3M™ Littmann® CORE™ Digital Stethoscope includes (1) CORE™ Digital Stethoscope, (2) Eartips, and (1) micro USB cable.
There is no detachable part on the CORE™ Digital Stethoscope and 3M™ Littmann® CORE™ Digital Stethoscope during normal operation.
Eko CORE™ Digital Attachment Compatible Stethoscopes
The Eko CORE™ Digital Attachment compatible hardware and software platforms are listed below.
CORE™ is designed and tested to be compatible with the 3M™ Littmann®
Cardiology III™, 3M™ Littmann® Cardiology IV™, WelchAllyn Harvey™ Elite®, Medline and ADC analog stethoscopes. CORE™ is compatible with many other stethoscope brands and models, but there are no performance guarantees when using other stethoscope brands or models.
NOTE: Eko CORE™ Digital Attachment is not compatible with Sprague stethoscopes or other digital stethoscopes.
Bluetooth and Data Connection
In order to transmit sounds to the Eko App, the stethoscope and device must be connected via Bluetooth, and in order to fully use certain functions, the mobile device must be connected to the internet via cellular data connection or Wi-Fi. Please keep CORE™ and Eko App within 15 feet for optimum Bluetooth connection. In the highly unlikely condition that the device is rebooted, revert to using the analog mode. The digital mode should restart in less than ten seconds.
System Requirements
The mobile app software can be used on iPhone 5S, iPhone 6/6 Plus, iPhone 6s/6s Plus, iPhone 7/ 7 Plus, iPhone 8/8 Plus, iPhone X, XS, XS Max, iPad* Mini 2/3/4, iPad Air/Air 2, iPad Pro, iPod Touch 6G, and iPad 5th and 6th generations with iOS 12.0 and higher. The mobile app software can also be used with Android devices with BLE support (Bluetooth 4.0) and Android 8.0 and above.
CORE™ uses Bluetooth Smart; mobile devices used must be compatible with Bluetooth Smart.
Installation to Existing Stethoscopes
This section is not required for pre-assembled CORE™ Digital Stethoscope and 3M™ Littmann® CORE Digital Stethoscope.

- Grip chest piece with one hand and pull the tubing with force using the other hand to detach the chest piece from the tubing of the existing stethoscope. Insert the chest piece into the Eko-compatible adapter tubing provided
- Attach the CORE™ Digital Attachment to the other end of the Eko-compatible adapter tubing provided
- Attach the tubing of the existing digital stethoscope to the other end of the CORE™ Attachment and assembly of the CORE™ digital stethoscope is now complete
CORE™ Use
Charge Battery
The battery in CORE™ will need to be charged; insert the included micro
USB cable into the USB port on the device and plug the other end into a UL-certified USB wall charger. The LED will turn solid yellow, signifying that it is charging. The LED will change to solid green when the device is fully charged. The fully charged battery should last for at least 8 hours in continuous transmission mode (ON, Bluetooth paired with Eko App).
NOTE: CORE™ will not turn on while it is plugged in and charging.
Power Off
When CORE™ is turned Off, analog rather than digital sounds will be transmitted and heard from the stethoscope. "OFF" is when the toggle is protruding from the surface of the volume buttons.
Power On
Depress the power slider to move the switch from the OFF to the ON position. "ON" is when the toggle is flush with the surface of the volume buttons.
Test the Volume Level
CORE™'s sound level can be amplified in 7 increments up to 40X amplification of an acoustic stethoscope. Change the volume level by clicking the plus (+) and minus (-) volume buttons on the side of CORE™.
Bluetooth Pairing
First, enable Bluetooth on the selected mobile device. On the iOS device go to Settings > Bluetooth > and tap the slider to turn Bluetooth ON. The mobile device is now ready to record sounds from CORE™. If Bluetooth pairing is unsuccessful, an error message will appear in the App and no sounds will be recorded. If the Bluetooth connection is successful the LED will turn from flashing white to solid white (See Section for the LED states of the device).
Setting up a PIN
Create a secure 4-digit PIN by logging in to the mobile application. Navigate to the Menu screen by selecting the icon on the top left of the Mobile App home screen.
Next, select Account Settings > Create Pin. Follow the instructions on the screen to create and save a 4 -digit PIN. You will need to enter your PIN twice for verification purposes.
Adding Notes to Recordings on Mobile App
To create notes on any patient recordings, log into the mobile application. Access the list of patients by selecting the patients tab on the top right of the home screen. Select the desired patient and select a recording to add notes to. On the bottom of the recording screen, select the Notes icon. The Notes icon looks like a Post-It® with writing on it. Select "Add Note" and begin typing your note. Select the check mark to save.
Operating the CORE™
When using the CORE™ to assess and record heart sounds, it is best to place the CORE™ stethoscope at the standard auscultation points on the anterior chest wall as shown below with BLACK dots (refer to Figure 4a). When using the CORE™ to assess and record lung sounds, it is best to place the CORE™ stethoscope at the standard auscultation points on the anterior chest wall as shown below with BOTH black and blue dots (refer to Figure 4).
The diaphragm side of the stethoscope should be placed on user's chest wall to assess for both heart and lung sounds. Only use the bell (or closed bell) of the stethoscope when assessing low frequency sounds as recommended by a clinician (refer to Figure 2).
Headset alignment
Before placing the eartips in your ears, hold the headset in front of you with the eartubes pointing away. Once the eartips are in your ears, they should point forward.
Open the diaphragm
When using a double-sided stethoscope (refer to Figure 3), you need to open (or index) the bell or diaphragm by rotating the chestpiece.
If the diaphragm is open, the bell will be closed, preventing sound from coming through the bell, and vice versa.


Cleaning
Cleaning Procedure
The Eko CORE™ Digital Stethoscope should be disinfected between each use. Infection control guidelines from the Centers for Disease Control and Prevention (CDC) state that reusable medical equipment, such as stethoscopes, must undergo disinfection between patients. Standard stethoscope hygiene practices apply to the Eko device.
All external parts of the hardware should be disinfected with 70% isopropyl alcohol wipes. Under normal conditions, it is not necessary to remove CORE™ attachment from the stethoscope tubing during the disinfecting procedure.
NOTE: DO NOT immerse the device in any liquid or subject it to any highpressure/autoclave sterilization processes.
It might be necessary to remove the Eko CORE™ Digital attachment from the Stethoscope, if the device is excessively soiled or a fluid invasion is suspected. The following disassembly procedure is recommended:,
- Pull the stethoscope tubing off of the metal stem of the CORE™ attachment on both ends.
- Wipe all parts of the stethoscope clean with 70% isopropyl alcohol wipes or disposable hospital-grade disinfectant wipe, or disposable wipes with soap and water including CORE™'s surface, stethoscope tubing, tubing connector, eartips and chest piece.
- A 2% bleach solution may be used to disinfect your stethoscope tubing, tubing connector, and chest piece; however, the tubing may become discolored after exposure to bleach.
- To prevent staining of stethoscope tubing, avoid contact with pens, markers, newsprint, or other printed material. It is good practice to wear your stethoscope over a collar whenever possible.
- Reassemble the stethoscope by reinserting the metal stems of the CORE™ attachment into the stethoscope tubing as described above in the installation section.
Operating Conditions
Environmental conditions of transport and storage between uses -40° to +55°C, relative humidity range of 15% to 93% at an atmospheric pressure range of 700 hPA to 1060 hPa (conforming to IEC 60601-1-11).
Continuous operating conditions
-30° to 40°C; relative humidity range of 15% to 93%, at an atmospheric pressure range of 700 hPa to 1060 hPa (conforming to IEC 60601-1-11) Avoid exposure to extreme heat, cold, solvents and oils. Extreme heats and colds will negatively affect the lithium ion battery in the device and may affect battery life.
No Modifications
Failure to follow care and maintenance recommendations could result in damage to the internal components of CORE™. Internal damage to the product could cause malfunction of the product, which may lead to complete loss of function. If problems are encountered with CORE™, do not attempt to repair it. Please notify our support team for assistance.
Additional processing between uses
Eko CORE™ Attachment should be sufficiently charged and disinfected between uses following instructions provided in this manual."
"Eko CORE™ Attachment should not be reused if
- The device enclosure or attachment has visible damage,
- The device does not power ON/OFF,
- The device cannot be sufficiently charged,
- The device exhibits acoustic issues,
- The device exhibits other operational anomalies."Contact Eko customer support for further assistance.
Serious Incident Reporting
If a serious incident has occurred in relation to the device, it should be reported to the manufacturer and the competent authority of the Member State in which the user and/or patient is established. A serious incident means any incident that directly or indirectly led, might have led or, in case of recurrence, could lead to any of the following: the death of a patient, user or other person, the temporary or permanent serious deterioration of a patent's, user's, fetus or other person's state of health, or a serious public health threat.
CORE™ Modes and Corresponding LED States
 | CORE is on & seeking device |
 | CORE is on & connected, CORE is recording |
 | CORE is playing back from a recording |
 | CORE is low on battery |
 | CORE is off & charging |
 | CORE is fully charged |
Eko App

Download the Eko app, available on the App Store® and Google Play and follow the on-screen instructions to connect to CORE™. Select Eko App features may require a paid subscription.
Bluetooth must be enabled in the mobile or desktop's Bluetooth settings in order to use CORE™ with the Eko App.
When using the Eko Dashboard and Eko App, enable device and networking security features to protect patient data that is created and stored using this software, in addition to security features embedded in the system. Update to the latest version of the Eko App.
Electrical Emission and Device
| Eko CORE Specifications | |
| General Performance | |
| Device expected service life (device and accessories) | 2 Years |
| Audio output | .WAV file |
| Audio A/D sampling rate | 4000 Hz |
| Frequency response | 20Hz-2000Hz |
| Max audio playback intensity | 100db |
| Bluetooth Characteristics | |
| Frequency band | 2400-2482MHz |
| Receiver sensitivity level | -95dBm |
| Operational distance | Up to 15 ft unobstructed |
| Power | |
| Battery type | Rechargeable 3.7 V Lithium-ion polymer cell |
| Battery life | ~10 hours continuous use, 1 week typical use |
| Physical Characteristics | |
| Dimensions | 71 mm long. 24 mm in diameter |
| Weight | 34 grams (capsule only) |
| Environmental Specifications | |
| Environmental conditions of transport and storage between uses | – 40°C to + 55°C, relative humidity range of 15% to 93% at an atmospheric pressure range of 700 hPa to 1060 hPa |
| Continuous operating conditions | -30°C to + 40°C; relative humidity range of 15% to 93%, at an atmospheric pressure range of 700 hPa to 1060 hPa |
| Standards Compliance | |
| Medical electrical equipment Part 1: General requirements for basic safety and essential performance | IEC 60601-1 |
| IEC 60601-1-2 | |
| IEC 60601-1-6 | |
| IEC 60601-1-11 | |
| User Interface | |
| Core device hardware | Hand-held device with LED, buttons, and micro USB port for charging using a certified USB-IF, Class II Double insulated USB charging port with output voltage rated at 4.75V-5.25V and charging current at 500mA - 2A. |
| Mobile device | iPhone or iPad with iOS 13.0 and above |
| PC | Windows OS or Mac OS X with supported web, browsers |
Clinical Benefits
The Eko CORE™ is a digital auscultation tool that improves the physical assessment of patients by providing sound amplification, active noise cancellation, and wireless listening.
The Eko CORE™ also includes a clinical decision support tool for automated murmur detection with the use of Eko Analysis Software (EAS) or Eko Murmur Analysis Software (EMAS) as available.
As an integral part of a physical assessment, clinicians' interpretations of body sounds via the Eko CORE™ and Eko AI outputs can help them rule in or out different pathological conditions in a patient.
By helping physicians accurately detect murmurs that warrant further investigation, further workup and testing could be better focused and more likely to result in the clinical benefit of an accurate diagnosis for the patient.
Network Security
When connecting your smart device, use a network that supports Wi-Fi 802.11n. It is recommended to secure this network using WPA (Wi-Fi Protected Access) or WPA2 (Wi-Fi Protected Access II) as your security protocol. For information on setting up your wireless network security, refer to your network equipment's documentation.
All data transmitted from the Eko App is encrypted in transit using TLS 1.2 or greater, and all data is encrypted at rest using AES 256.
In addition to security features embedded in the system, it's highly recommended that users of the Eko App and Eko Dashboard use networking security features to protect patient data created and stored using this software. Common examples include strong passwords, biometric authorization, twofactor authentication, and VPN encryption when available.
CORE™ supports the use of Bluetooth as the primary communication protocol to the mobile device during operation. Bluetooth is a short-range wireless technology standard using UHF radio waves in the ISM bands, from 2.402 to 2.48 GHz.
Firmware updates to the CORE™ will be made available as over-the-air (OTA) updates through your Eko App on mobile devices. Eko provides regular updates for your Eko App, available through the mobile device app store.
Cybersecurity
Eko is committed to safeguarding device cybersecurity by establishing an active cybersecurity monitoring program. The CORE™ device does not perform cybersecurity event detection nor event logging for cybersecurityrelated events.
Eko has established instructions for users or user facilities regarding network and connection requirements. Refer to https://support.ekohealth.com
Users are encouraged to review the instructions for any security actions that the user or user facility are expected to implement to ensure secure use of the CORE™ device. Refer to information available on https://support.ekohealth. com regarding Eko Administration and IT Support.
If a cybersecurity event has been detected or suspected, please report to security@ekohealth.com and privacy@ekohealth.com.
Patient Privacy
The privacy of patient health information may be protected by state, federal, or international/foreign laws that regulate how such information can be used, stored, transmitted, and disclosed. The Eko system employs security features that are compliant with HIPAA policies. Third-party access may be prohibited to such information without obtaining written authorization from the patient.
The user is fully responsible for understanding and following all laws that regulate storage, storage transmission, and disclosure of any electronic patient data through the use of software. If the user becomes unable to comply with a law or restriction that applies to use and disclosure of such data, the user should not proceed to collect or save such information.
This application may require entry of individually identifiable health information in order to function. Records are stored and recalled through the use of patient name, date of birth, and/or patient ID number. By entering this information, the user assumes any and all risks of and liabilities incurred with using or transmitting such information.
If a suspected cybersecurity event has occurred, please report to security@ekohealth.com and privacy@ekohealth.com
Documents / Resources
References
Download manual
Here you can download full pdf version of manual, it may contain additional safety instructions, warranty information, FCC rules, etc.
Advertisement










Need help?
Do you have a question about the CORE and is the answer not in the manual?
Questions and answers