EKO Core Manual
Hide thumbs
Also See for Core:
- User manual ,
- Quick start manual (68 pages) ,
- Quick start manual (2 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for EKO Core
- Page 1 Model 2nd Generation © 2020 Eko Devices, Inc.
-
Page 2: Indications For Use
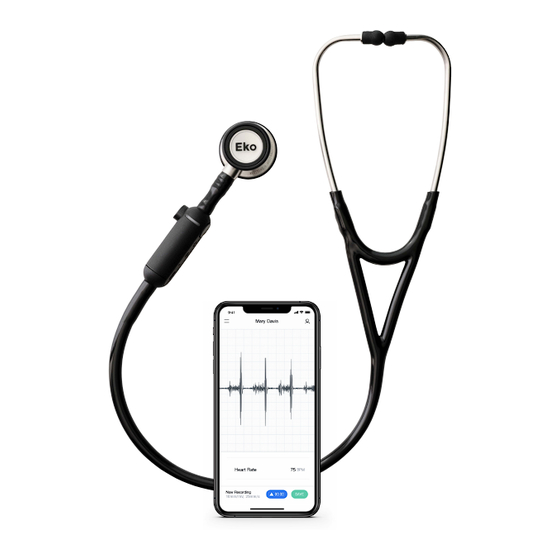
(heart, lungs, bowel, arteries, and veins), whereby a clinician at one location on network can listen to the auscultation sounds of a patient on site or at a different location on the network. Eko CORE is intended for use on pediatric and adult patients. The Eko CORE is intended to be used by professional users in a clinical environment or by lay users in a nonclinical environment. - Page 3 Eko device also enables regular users to record, store and share their body sounds with their physician. Eko CORE includes a device that is attached to a stethoscope (CORE attachment) and an application, the Eko App. CORE features sound amplification and audio transmission to...
-
Page 4: Equipment Symbols
4. Equipment Symbols Instructions for use European technical conformity 0537 European Authorized Representative EC REP Do not dispose with household waste Emits Radio Frequency signal Model number Humidity range Temperature range Non-sterile device. Do not attempt to re-sterilize Wireless Bluetooth communication Manufacturer Manufacturing date Quantity... - Page 5 Eko Devices, Inc. may result in increased RF emissions or decreased immunity of the Eko CORE. Please read, understand, and follow all safety information contained in these instructions prior to using the Eko CORE. It is recommended that these instructions be retained for future reference.
- Page 6 To improve Bluetooth connection, reduce the distance and/or allow a line of sight between Eko device and mobile device. The Bluetooth range will be reduced when objects (walls, furniture, people, etc) are between the Eko device and a paired mobile device.
-
Page 7: Emc Compliance
6. EMC Compliance FCC Intentional Radiator Certification Contains FCC ID: 2ANB3-E6 Contains IC: 23063-E6 47 CFR Part 15.105 required statement for Class B: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. - Page 8 NO MODIFICATION Modifications to this device shall not be made without the written consent of Eko Devices, Inc. Unauthorized modifications may void the authority granted under Federal Communications Commission rules permitting the operation of this device.
- Page 9 (1) micro USB cable and the Eko App. The compatible hardware and software platforms are listed below. Compatible Stethoscopes Eko CORE is designed and tested to be compatible with the 3M Littmann* Cardiology II/III/IV, WelchAllyn Harvey Elite, Medline and ADC analog stethoscopes. Eko CORE is compatible with...
- Page 10 Android devices with BLE support (Bluetooth 4.0) and Android 7.0 and above. CORE uses Bluetooth Smart; mobile devices used must be compatible with Bluetooth Smart. *Littmann, 3M , and Cardiology III are registered trademarks of the 3M Corporation.
- Page 11 Attach the CORE Digital Attachment to the other end of the Eko-compatible adapter tubing provided Step Three Attach the tubing of the existing digital stethoscope to the other end of the CORE Attachment and assembly of the CORE digital stethoscope is now complete Figure 2...
- Page 12 9. CORE Use Charge Battery The battery in CORE will need to be charged; insert the included micro USB cable into the USB port on the device and plug the other end into a UL-certified USB wall charger. The LED will turn solid yellow, signifying that it is charging.
- Page 13 Note” and begin typing your note. Select the check mark to save. Operating CORE When using the Eko CORE to assess and record heart sounds, it is best to place the CORE stethoscope at the standard auscultation points on the anterior chest wall as shown below with BLACK dots (refer to Figure 4).
- Page 14 Figure 4 Headset alignment Before placing the eartips in your ears, hold the headset in front of you with the eartubes pointing away. Once the eartips are in your ears, they should point forward. Open the diaphragm When using a double-sided stethoscope (refer to Figure 3), you need to open (or index) the bell or diaphragm by rotating the chestpiece.
- Page 15 If it becomes necessary to remove CORE, pull the stethoscope tubing off of the metal stem of the CORE attachment on both ends. Wipe all parts of the stethoscope clean with 70% isopropyl alcohol wipes or disposable wipe with soap and water including CORE’s surface, stethoscope tubing, tubing connector, and...
-
Page 16: Operating Conditions
11. Operating Conditions Environmental The operating range of CORE is -30° to 40°C (-22° to 104°F), and 15% to 93% relative humidity. The storage and transport range is -40° to 55°C (-40° to 131° F), and 15% to 93% relative humidity. - Page 17 CORE is off & charging CORE is fully charged 14. Eko App Download the Eko app, available on the App Store® and Google Play and follow the on-screen instructions to connect to CORE (as shown on the next two pages).
- Page 18 14. Eko App: Setup and Pairing ➊ ➋ Sign up: Physician setup: Enter names and email address Enter physician email address ➌ ➍ Turn on Eko CORE Pair Eko CORE...
- Page 19 14. Eko App: Recording ➎ ➏ Settings Start recording ➐ ➑ Recording in progress Recording complete...
-
Page 20: Electrical Safety
Guidance and Manufacturer’s Declaration - Electromagnetic Emission The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment specified below. The user of the Eko Electronic Stethoscope System should assure that it is used in such an environment. - Page 21 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment specified below. The user of the Eko Electronic Stethoscope System should assure that it is used in such an environment.
- Page 22 To address the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Eko Electronic Stethoscope System is used exceeds the applicable RF compliance level above, the Eko Electronic Stethoscope System should be observed to verify normal operation.
- Page 23 Electronic Stethoscope System The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The user of the Eko Electronic Stethoscope System can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Eko Electronic Stethoscope System as recommended below, according to the maximum output power of the communications equipment.
- Page 24 Oakland, CA 94612 USA www.ekohealth.com 0537 Notified Body: Eurofins Expert Services Oy Notified Body No. 0537 EC Authorized Representative: Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands LBL 071, Rev C - Issued Date: May 2020 © 2020 Eko Devices, Inc.









Need help?
Do you have a question about the Core and is the answer not in the manual?
Questions and answers