Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Nouvag LipoSurg
- Page 1 Instructions for use LipoSurg...
- Page 2 INSTRUCTIONS FOR USE CONGRATULATIONS ON YOUR PURCHASE OF A PRODUCT FROM NOUVAG. We are pleased that you have chosen a quality product from NOUVAG and thank you very much for the trust you have placed in us. These instructions for use will familiarize you with the device and its functions so that you can apply and use them correctly.
-
Page 3: Table Of Contents
Tubing set REF 6022 and REF 6026 MAINTENANCE Replacing the control unit fuses Safety inspections MALFUNCTIONS AND TROUBLESHOOTING ACCESSORIES AND SPARE PARTS Information on disposal TECHNICAL DATA WARRANTY COVERAGE Post market surveillance Service points APPENDIX © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 4: Product Description
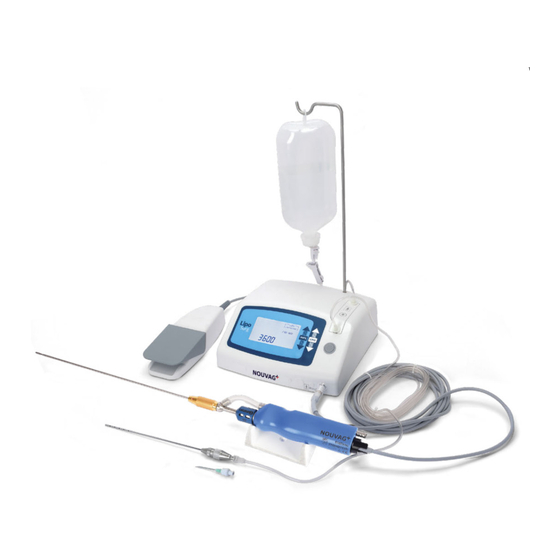
PRODUCT DESCRIPTION INTENDED USE AND OPERATION The LipoSurg is a useful and precisely matched combination of devices consisting of a peristaltic pump for infiltration of tumescent fluid and a motor drive for moving the liposuction cannula. This allows a tissue-gentle liposuction with or without tumescent local anesthesia. -
Page 5: Safety Information
INSTRUCTIONS FOR USE LIPOSURG SAFETY INFORMATION It is essential to bear the following information in mind Every use of the LipoSurg different to the product descrip- tion defined in section causes risks for patients and trained personnel. If physical [ Intended use and operation > 4 ] examinations and therapies are carried out without use of the devices, then the devices must be removed from the place of treatment. -
Page 6: Modification And Misuse
INSTRUCTIONS FOR USE SAFETY INFORMATION MODIFICATION AND MISUSE Modification or manipulation of the LipoSurg and its accessories is prohibited. The manufacturer is not liable for any damages resulting from unauthorized modifications or manipulations. The warranty will be canceled. Use of the LipoSurg outside the indications described in is prohibited. -
Page 7: Scope Of Delivery
Clip set, for tube set attachment to motor cable, PU 5 pcs. 1770 Stand for irrigation fluid bottle 1170 Handpiece tray 19584 Spray adapter with thread, for lubricant spray (REF 2128) 31648 LipoSurg instructions for use © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 8: Device Overview
Connection for potential equaliza- tion Power plug socket Main switch Fuse compartment Spray nozzle for maintenance of Conform cannula handpiece © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 9: Setup
SETUP DEVICE AND ACCESSORIES SETUP ¬ Place the LipoSurg and all required accessories and instruments on an even, non-slip surface and make sure you have good access to all controls. ¬ The installation of the device near other devices is prohibited due to EMC. -
Page 10: Device Preparation
Cannula adapter with suction channel (REF 75705) Connection tube (REF 29063) Protec- tive cap (REF 40376) Silicone sealing (REF 40377) Conform cannula handpiece (REF 5107) Closure 8 Electronic motor (REF 2101nou) Suction pipe integrated in handle Handle © NOUVAG AG • 31648 • V20230330 • All rights reserved... - Page 11 Now screw the cap past the cable into the open- Seat the silicone sealing via the tread onto the ing of the Conform cannula handpiece. Conform cannula handpiece. © NOUVAG AG • 31648 • V20230330 • All rights reserved...
- Page 12 Connect the connection tube to the suction pipe. Screw the cannula onto the cannula adapter. Adjust the alignment of the hose by loosening the closure on the back of the handpiece. © NOUVAG AG • 31648 • V20230330 • All rights reserved...
- Page 13 The integrated peristaltic pump is used for the infiltration of the tumescent solution. Do not regulate the amount of irrigation fluid using the roller clamp on the tubing set; with the LipoSurg, this is regulated instead using the integrated pump. For this reason, make sure to open the roller clamp as far as it will go (refer also to [ Overview: Control elements >...
-
Page 14: Operation
By pressing the « LIPO » key, the number of cannula strokes is reduced. By pressing both « INFIL » and « LIPO » keys simultaneously, the « COMBI-MODE » is activated. In this case INFIL and LIPO are both active at the same time. © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 15: Infiltration " Infil
Selected function « INFIL & LIPO » By pressing both « INFIL » and « LIPO » keys simultaneously, the « COMBI-MODE » (Infiltration & Liposuction) is displayed. Set values for both functions just as mentioned above. © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 16: Operation With Vario Pedal
Pump runs with the speed shown by the bar graph « ON/OFF » PEDAL MODE STEP PLATE... CANNULA STROKE PERISTALTIC PUMP No cannula stroke Pump off not pressed pressed shortly Cannula works with preset value Pump runs with preset value © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 17: Functional Check
FUNCTIONAL CHECK Prior to every startup of the LipoSurg, or the use of accessory equipment, the user must always ensure that each individual component is in good working order, free from defects, and is clean, sterile and operational. All inscrip- tions on the device and its accessories must be readable and there must be no loose parts in the device. -
Page 18: Cleaning And Sterilization
Don’t use tube set when packaging is already opened or damaged! Do not use tubing set if expired. Use only NOUVAG tubing sets with REF 6022 and REF 6026. Sterility cannot be guaranteed by reusing and re-sterilization of tubing sets. The characteristics of the mate- rial may change resulting in serious infections or, in worst case, the death of the patient. -
Page 19: Maintenance
Results shall be documented. The service manual, wiring diagrams, and descriptions are available upon request from the manufacturer. NOUVAG offers a safety inspection service for its customers. Addresses can be found in the appendix of this instructions for use under . -
Page 20: Malfunctions And Troubleshooting
If the problem cannot be solved please contact your supplier or an authorized service center. Addresses can be found in the appendix of this instruc- [ Service points > 24 ] tions for use under © NOUVAG AG • 31648 • V20230330 • All rights reserved... - Page 21 Nou-Dongle is plugged in This message is displayed for one second when the Nou dongle is plugged in. Fatal error! E38, Wrong hardware System Message XX Send unit to service point © NOUVAG AG • 31648 • V20230330 • All rights reserved...
-
Page 22: Accessories And Spare Parts
Power cord DE, with device socket, 3 m 22262 Power cord GB, with device socket, 3 m 22264 Power cord US, with device socket, 3 m 22266 To order additional parts, please contact our customer service department. © NOUVAG AG • 31648 • V20230330 • All rights reserved... -
Page 23: Information On Disposal
Dimensions (W x D x H) 260 x 250 x 110 mm Net weight control unit 3,4 kg * Applied parts are the instruments used with the LipoSurg. ELECTRONIC MOTOR TO DRIVE THE CANNULA PERISTALTIC PUMP Motor coupling INTRA coupling, ISO 3964 Volumetric displacement 1 –... -
Page 24: Warranty Coverage
6 months, wear parts are excluded from the warranty. During this warranty period, NOUVAG agrees to either repair or replace the product at its option if the product fails to function properly under normal use and service and such failure is due solely to a defect in workmanship or materials. -
Page 25: Appendix
Electromagnetic compatibility (EMC) Remark: The Product subsequently referred to herein always denotes the LipoSurg. Changes or modifications to this product not expressly approved by the manufacturer may result in increased emissions or decreased immunity performance of the product and could cause EMC issues with this or other equipment. This product is designed and tested to comply with applicable regulations regarding EMC and shall be installed and put into service according to the EMC information stated as follows. - Page 26 Product should b observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Product. over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m. © NOUVAG AG • 31648 • V20230330 • All rights reserved...
- Page 27 At 80 MHz and 800 MHz, the separation distance fort the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. © NOUVAG AG • 31648 • V20230330 • All rights reserved...
- Page 28 NOUVAG AG NOUVAG GmbH St. Gallerstrasse 25 Schulthaissstrasse 15 9403 Goldach 78462 Konstanz Switzerland Germany Phone +41 71 846 66 00 Phone +49 7531 1290 - 0 info@nouvag.com info-de@nouvag.com www.nouvag.com www.nouvag.com © NOUVAG AG • 31648 • V20230330 • All rights reserved...
















Need help?
Do you have a question about the LipoSurg and is the answer not in the manual?
Questions and answers