Table of Contents
Advertisement
Quick Links
Defibtech Automated External Defibrillator
•
Lifeline/ReviveR VIEW AUTO DDU-2200
•
Lifeline/ReviveR VIEW DDU-2300
•
Lifeline/ReviveR ECG DDU-2450
•
Lifeline/ReviveR ECG+ DDU-2475
User Manual
For comprehensive training on set-up,
use and maintenance; source for
complete technical specifications
ELECTRONIC
DISTRIBUTION
DAC-U2510EN-BF rev H
Advertisement
Chapters
Table of Contents
Troubleshooting

Summary of Contents for Defibtech Lifeline/ReviveR ECG+ DDU-2475
- Page 1 Defibtech Automated External Defibrillator • Lifeline/ReviveR VIEW AUTO DDU-2200 • Lifeline/ReviveR VIEW DDU-2300 • Lifeline/ReviveR ECG DDU-2450 • Lifeline/ReviveR ECG+ DDU-2475 User Manual For comprehensive training on set-up, use and maintenance; source for complete technical specifications ELECTRONIC DISTRIBUTION DAC-U2510EN-BF rev H...
- Page 2 PRESS “ON” BUTTON APPLY PADS FOLLOW AED INSTRUCTIONS DAC-U2510EN-BF rev H...
- Page 3 “DDU-2000 Series. ” Notices Defibtech, L.L.C. shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance, or use of this material. Information in this document is subject to change without notice. Names and data used in any examples are fictitious unless otherwise noted.
-
Page 4: Table Of Contents
3.3 Installing And Removing The Battery Pack ..............15 3.4 Checking The DDU-2000 Series AED Status ..............16 3.5 Installing The Defibtech Data Card (DDC Card) (optional) ..........17 3.6 Completing The Installation ..................... 17 3.7 Storing The DDU-2000 Series AED ................. 17 Using The DDU-2000 Series In AED Mode ............18... - Page 5 7 .5 USB Cable ........................48 Event Viewing ....................49 8.1 DefibView ........................49 8.2 Defibtech Data Cards (DDC Cards) ................. 49 8.3 Downloading The Internal Data Log ................49 Technical Specifications ..................50 9.1 Defibtech DDU-2000 Series AED ..................50 9.2 Battery Packs ........................59 9.3 Self-Adhesive Defibrillation Pads ..................
-
Page 6: Introduction To The Ddu-2000 Series Aed
Introduction To The DDU-2000 Series AED This User Manual provides information to guide operators in the use and maintenance of the Defibtech DDU-2000 Series Automated External Defibrillator (AED) and its accessories. It includes comprehensive training on set-up, use, and maintenance and is the source for complete technical specifications. - Page 7 Each battery pack is marked with an expiration date. The DDU-2000 Series AED records event documentation internally and, optionally, on a Defibtech Data Card (DDC). The optional DDC card inserts into a slot in the AED and enables the AED to record event documentation and, optionally, audio data onto the card.
-
Page 8: The Defibtech Ddu-2000 Series Aed
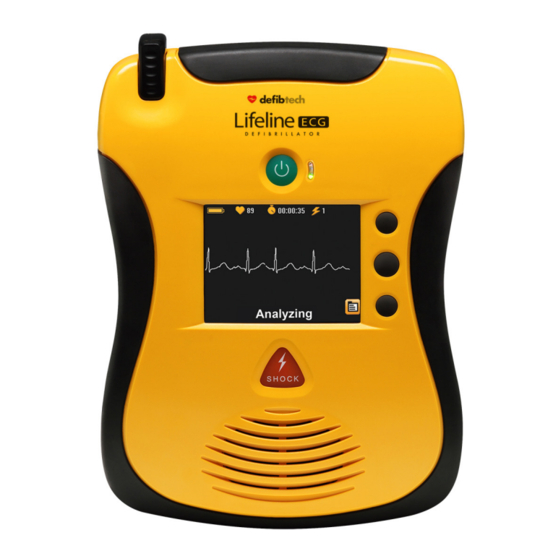
The speaker also emits a “beep” when storage capabilities to the AED. the unit is off and has detected a condition J. USB and Defibtech Data Card (DDC card) that requires attention from the user or Access Door. Behind the access door is the needs servicing. - Page 9 DDU-2200) H. USB Port A. Speaker I. Defibtech Data Card (DDC Card) (optional) J. USB and Defibtech Data Card (DDC Card) Access Door FRONT OF AED K. Battery Pack Opening L. Battery Pack Eject Release Latch M. Pad Storage Area N.
-
Page 10: Usage
Lifeline/ReviveR ECG+ DDU-2475 AEDs may be used with Defibtech adult defibrillation pads (model number DDP- 2001). For patients under 8 years old, or weighing less than 55 lbs (25 kg), use Defibtech child/infant defibrillation pads (model number DDP-2002), if available. -
Page 11: Warnings And Cautions
Warnings And Cautions This chapter includes a list of warning and caution messages that relate to the Defibtech DDU-2000 Series AED and its accessories. Many of these messages are repeated elsewhere in this User Manual and on the DDU-2000 Series AED or accessories. - Page 12 WARNINGS (continued) • Do not allow defibrillation pads to touch each other, or to touch other ECG electrodes, lead wires, dressings, transdermal patches, etc. Such contact can cause electrical arcing and patient skin burns during defibrillation and may divert defibrillating energy away from the heart. •...
-
Page 13: Cautions
• If possible, disconnect the DDU-2000 Series AED from the patient prior to use of other defibrillators. • Using non-Defibtech Data Cards (DDC cards) may damage the unit and will void the warranty. • DefibView software is not intended for clinical use. Information presented by DefibView should not be used for making clinical decisions. -
Page 14: Setting Up The Ddu-2000 Series Aed
Setting Up The DDU-2000 Series AED This chapter describes the steps required to make your Defibtech DDU-2000 Series AED operational. The DDU-2000 Series AED is designed to be stored in a “ready” state. This chapter tells you how to make the device ready, so that if and when you need it, few steps are required to begin using the device. -
Page 15: Connecting The Defibrillation Pads
3.2 Connecting The Defibrillation Pads The DDU-2000 Series AED defibrillation pads are supplied sealed in a package with the connector and part of the cable exposed. DO NOT open the sealed pads package until the pads are to be used. The packaging should be opened only immediately prior to use, otherwise the pads may dry out and become non-functional. -
Page 16: Checking The Ddu-2000 Series Aed Status
• Solid Green: The DDU-2000 Series AED is ON and ready for use. • Flashing or Solid Red: The DDU-2000 Series AED needs immediate service. Refer to the “Troubleshooting” section in Chapter 5 of this manual or call Defibtech for service. Active Status •... -
Page 17: Installing The Defibtech Data Card (Ddc Card) (Optional)
3.5 Installing The Defi btech Data Card (DDC Card) (optional) The Defi btech Data Card (DDC card) is used to store event and audio information collected by the AED. All DDU-2000 Series AEDs will operate without DDC cards and will still store select event information internally. Information stored on the DDC card is retrievable with a separate Defi btech PC-based software package. -
Page 18: Using The Ddu-2000 Series In Aed Mode
Using The DDU-2000 Series In AED Mode This chapter describes how to use the DDU-2000 Series in AED Mode. In AED Mode, the unit analyzes the patient’s rhythm and automatically charges if a shockable rhythm is detected. Push-button controls include an ON/OFF button and three softkey buttons;... - Page 19 Overview (continued) Unit Video Display Screen (During AED Mode with video) Battery Indicator Rescue Breathing Options Softkey Icon Information Softkey Icon Main Screen Mode Select Softkey Icon Text Prompts (DDU-2450 only) Give 30 Compressions (sample display screen) Battery Indicator – The Battery Indicator indicates the approximate remaining battery capacity. Main Screen –...
-
Page 20: Preparation
Unit ECG Display Screen (During AED Mode with ECG, DDU-2450 only) Battery Indicator Main Screen Mode Select Softkey Icon Analyzing Text Prompts (sample display screen) Battery Indicator – The Battery Indicator indicates the approximate remaining battery capacity. Heart Rate Indicator – The Heart Rate Indicator displays the patient’s heart rate. NOTE: There are no alarms issued by the AED related to the patient's heart rate. - Page 21 Selecting an Alternate Spoken Language Some AED models are factory confi gured to support an alternate spoken language. If the AED supports an alternate language, a Language Softkey Icon (represented in the form of a fl ag) will be displayed. When the Language Softkey Icon is present, the user may press the corresponding softkey to switch the spoken voice prompts to the alternate language.
- Page 22 Applying The Defi brillation Pads To The Patient Follow this procedure to apply the defi brillation pads to the patient: 1. Tear the defi brillation pads package along the dotted line near the top of the package. 2. Remove the pads from the package and follow the directions and diagram showing proper pad placement located on the pads package.
-
Page 23: Heart Rhythm Analysis
Follow DDU-2000 Series AED Instructions At this point, the DDU-2000 Series AED will check to make sure that the pads are well connected to the patient and that an adequate ECG signal is being received. Do not touch the patient. Eliminate any patient movement, and cease CPR at this time. If there is a problem with the pad connection, socket connection, patient motion, or other interference, the AED will guide the operator with both audible and displayed instructions. -
Page 24: Post-Use Procedures
4.6 Post-Use Procedures After the DDU-2000 Series AED has been used on a patient, the unit should be cleaned following procedures in the “Cleaning” section in Chapter 5 of this manual and prepared for the next use. The following steps should be performed: 1. - Page 25 Pad Connection/Pad Application-Related Prompts (continued) Voice Text “Peel adhesive pads from blue liner” Peel Pads Purpose: This instructs the user to peel each pad from the blue liner before placing the pads on the patient. Peel the pads from the blue liner only when the pad is ready to be placed. Place the pads with the sticky side of the pad on the patient’s bare skin.
- Page 26 Heart Rhythm Analysis Prompts Voice Text “Analyzing heart rhythm” Analyzing Rhythm “Analyzing” Analyzing Purpose: The DDU-2000 Series AED is actively analyzing the patient’s ECG signal. The AED will continue analyzing until it has determined whether a rhythm is shockable or non-shockable or if analyzing is interrupted for some reason. “Do not touch the patient”...
- Page 27 Shock-Related Prompts (continued) Voice Text “Shock cancelled” Shock Cancelled Purpose: The DDU-2000 series AED has aborted shock mode. If the unit detects a rhythm change to a non- shockable rhythm, the unit will cancel the shock. Also, on semi-automatic AEDs, if the shock button is not pressed within 30 seconds of the initial “press flashing SHOCK button”...
-
Page 28: Operational Environment
4.8 Operational Environment The Defibtech AED is designed to operate in a wide range of environmental conditions. To ensure the reliability and safety of the AED in a given environment, refer to the “Environmental” section in Chapter 9 of this manual for a detailed list of approved environmental conditions. -
Page 29: Maintenance And Troubleshooting
Maintenance and Troubleshooting This chapter describes the maintenance and troubleshooting procedures for the DDU-2000 Series AED. The self- tests that are automatically performed by the device are described along with recommended routine maintenance. A troubleshooting guide is provided to help diagnose user serviceable problems. The DDU-2000 Series AED contains no user serviceable parts. - Page 30 Checking The AED Status Using The AED Status Screen You may also check the status of the unit when it is off by pressing the center softkey button to enter Maintenance Mode and display the AED Status Screen. The AED Status Screen shown at left is used to provide a quick overview of the DDU-2000 Series AED’s status and to display select information without turning the unit on in AED Mode.
- Page 31 Checking The Condition Of The Unit And Accessories Inspect the unit for dirt and contamination, especially in the pads connector socket and around the battery pack opening. (Refer to the “Cleaning” section in this chapter of this manual for guidance on cleaning your AED.) Inspect the unit display screen for damage.
- Page 32 Checking Pad And Battery Pack Expiration Dates It is important to check the expiration dates of the pads and battery pack. Both may be checked when the unit is off by pressing the center softkey button to display the AED Status Screen. (Refer to the “AED Status Screen” section in Chapter 6 of this manual.) The expiration dates can also be found on each item: the pads package expiration date is printed on the outside of the sealed package;...
-
Page 33: Self-Tests
5.2 Self-Tests The DDU-2000 Series AED provides for both automatic and manually initiated self-tests. These self-tests test various components of the AED, including the system controls, battery pack condition, charge/discharge functions, and measurement and signal acquisition functions. Automatic Unit Self-Tests Every time the unit is turned on, power-on self-tests are performed to test the basic operation of the unit. -
Page 34: Operator's Checklist
“Routine Unit Maintenance” section of this chapter. As each item is completed it should be checked off. Defibtech DDU-2000 Series Operator’s Checklist Defibtech DDU-2000 Series AED Serial Number: ___________________________________________________ Defibtech DDU-2000 Series AED Location:... -
Page 35: Troubleshooting
5.6 Troubleshooting The following table lists the symptoms, the possible causes, and the possible corrective actions for common problems. Refer to the other sections of the user manual for detailed explanations on how to implement the corrective actions. If the unit continues to be non-functional, refer the unit for servicing. (Refer to Chapter 12 of this manual for contact information.) Symptom Possible Cause... -
Page 36: Repair
“ Replace data card” prompt DDC card has failed Replace DDC card 5.7 Repair The DDU-2000 Series AED contains no user serviceable parts. If the unit needs servicing, call Defibtech. (Refer to Chapter 12 of this manual for contact information.) DAC-U2510EN-BF rev H... -
Page 37: Maintenance Mode
Maintenance Mode 6.1 Overview Maintenance Mode for the Defi btech DDU-2000 Series AED permits the user to perform maintenance-related actions such as viewing unit information, initiating unit self-tests, changing unit parameters, downloading rescue data, and upgrading software. Maintenance Mode is navigated through a series of screens, menus, and menu options. In Maintenance Mode, the softkey buttons located directly to the right of the display screen are used to scroll through and select menu options. -
Page 38: Entering Maintenance Mode
6.3 Entering Maintenance Mode Before You Begin: Make certain the DDU-2000 Series AED is turned off and a battery pack is installed. STEP 1 – Press and release the CENTER softkey button. Result – The unit will turn on and display the AED Status Screen for a AED status short period of time. -
Page 39: Aed Maintenance Screen
AED Maintenance Screen The AED maintenance screen allows the user to select such options as AED tests, software upgrades, data backups, and data card functions. Before you begin: Be sure the unit is in Maintenance Mode. To enter: Navigate to AED maintenance: AED Main Menu Ú... - Page 40 Transfer Data To Card Transfer data to card will initiate a data transfer from the DDU-2000 Series AED to a Defibtech Data Card (DDC card) inserted in the device. Internal event data and device history is written to the DDC card.
- Page 41 Note: Formatting the data card will erase all existing data on the data card. Before you begin: Be sure the unit is in Maintenance Mode. Be sure a Defibtech Data Card (DDC card) is currently installed in the device. (Refer to the “Installing The Defibtech Data Card (DDC card)”...
-
Page 42: Aed Options Screen
Note: If a data card is not inserted, the unit will speak and display “Data Card Missing. ” (Refer to the “Installing The Defibtech Data Card (DDC card)” section in Chapter 3 of this manual.) To exit: When the unit finishes performing the application, follow the displayed and spoken instructions. - Page 43 Audio Recording The Audio recording option allows the user to enable or disable recording of event audio data to a Defibtech Data Card (DDC card). Before you begin: Be sure the unit is in Maintenance Mode. To enter: Navigate to Audio recording: AED Main Menu Ú...
-
Page 44: Rescue Options Screen
Status Broadcast* The Status broadcast option allows the user to enable or disable wireless (RF) transmission of status data to an optional data receiver (contact your Defibtech distributor for details). Before you begin: Be sure the unit is in Maintenance Mode. - Page 45 CPR cycles, and number of shocks between CPR). This code is custom generated by Defibtech. If the code is not entered correctly the protocol will not be changed. Based on the protocol code entered, the currently selected protocol will be changed to that described by the special protocol code.
-
Page 46: Help Topics Screen
Help Topics Screen The Help topics option on the AED Main Menu provides a list of available help topics. Before you begin: Be sure the unit is in Maintenance Mode. To enter: Navigate to Help topics: AED Main Menu Ú Help topics What it does: The Help topics provides a list of available help topics. -
Page 47: Ddu-2000 Series Aed Accessories
The DDC card is inserted into a slot behind the data card/USB port access door found on the side of the AED. (Refer to the “Installing The Defibtech Data Card (DDC card)” section in Chapter 3 of this manual.) A new event file is created on the DDC card each time the AED is turned on, and the following information is recorded: •... -
Page 48: Usb Cable
An optional USB cable may be used with the DDU-2000 Series AED for connecting the AED to a personal computer running Defibtech maintenance software. The AED has a mini-USB connector located on the right side of the unit behind the data card/USB port access door. -
Page 49: Event Viewing
Event Viewing This chapter includes information about DefibView, Defibtech Data Cards (DDC cards), and downloading internal data logs. 8.1 DefibView DefibView is a Windows-based software application that reads data stored on a DDC card or downloaded through the USB port and displays the data on a personal computer. DefibView serves the following primary functions: •... -
Page 50: Technical Specifications
Technical Specifications 9.1 Defibtech DDU-2000 Series AED General Category Specification Size 7 .3 x 9.5 x 2.3 inches (18.5 x 24 x 5.8 cm) Weight Less than 3 lbs (1.4 kg) (with battery) Power Battery Pack (not rechargeable) Meets applicable requirements of •... - Page 51 Defibtech DDU-2000 Series AED (continued) Defibrillator – ECG Display (DDU-2450 only) Category Specification ECG information is received from defibrillation pads in anterior-lateral or Displayed ECG anterior-posterior positions Screen type TFT Color LCD with backlight (53.6mm x 71.5mm) (320 x 240 pixels) Displayed Range Differential: ±2 mV full-scale...
- Page 52 Defibtech DDU-2000 Series AED (continued) Waveform Specifications The DDU-2000 Series AED delivers a 150 J (Adult) 50 J (Pediatric) Biphasic Truncated Exponential waveform (AED Mode) to patients with impedances ranging from 25 to 180 ohms. V max V min The waveform is adjusted to compensate for measured patient impedance.
- Page 53 Defibtech DDU-2000 Series AED (continued) Environmental Category Specification Temperature 0 – 50°C (32 – 122°F) Humidity 5% – 95% (non-condensing) Operating/ One Hour Operating Maintenance Temperature Limit -20°C (-4°F) (extreme cold)* Air Pressure 700 to 1060 hPa (21 to 31 inHg) Temperature 0 –...
- Page 54 DDU-100 and DDU-2000 series AEDs and the Philips defibrillation waveforms that were also used for the original clearance of the Defibtech AEDs. The waveform delivered by the Defibtech and Philip AEDs is a biphasic truncated, impedance-compensating exponential waveform. The comparison consisted of oscilloscope captures of the defibrillation waveforms, as shown in the examples below.
- Page 55 Philips AEDs or 200 to 360J monophasic waveform AEDs on victims where defibrillation was indicated. As noted above, the data provided by Defibtech demonstrates that the waveforms from Philips and Defibtech are almost identical. Therefore, the clinical data for adult defibrillation included in the Schneider publication was leveraged to support the safety and effectiveness of the Defibtech waveform.
- Page 56 Defibtech defibrillation waveform, the data provided by Defibtech demonstrates that the pediatric waveforms from Philips and Defibtech are almost identical. The Tang animal study tested the defibrillation waveform used for defibrillation success and safety on a pediatric model (swine). The Atkins clinical study was published in 2005 and evaluated the same waveform in a post-market study.
- Page 57 Ventricular Tachyarrhythmia), AHA (American Heart Association, Ventricular Arrhythmia), and Defibtech’s internal library of real-life and electronically-manipulated ECG recordings. The Defibtech AEDs meet the recommendations of the AHA12 and IEC 60601-2-4 for performance goals of arrhythmia analysis algorithms. The performance of the arrhythmia analysis algorithm is summarized in the following table.
- Page 58 References 1 Thomas Schneider, Patrick R. Martens, Hans Paschen, Markku Kuisma, Benno Wolcke; Bradford E. Gliner, James K. Russell, W. Douglas Weaver, Leo Bossaert, Douglas Chamberlain, for the Optimized Response to Cardiac Arrest (ORCA) Investigators. Multicenter, Randomized, Controlled Trial of 150-J Biphasic Shocks Compared With 200- to 360-J Monophasic Shocks in the Resuscitation of Out-of-Hospital Cardiac Arrest Victims.
-
Page 59: Battery Packs
Standby-life (installed in unit) 4 years* *Typical, new battery, at 25°C Note: For information about optional rechargeable lithium-ion battery packs, please visit www.defibtech.com or contact Defibtech or your distributor. 9.3 Self-Adhesive Defibrillation Pads Defibtech self-adhesive defibrillation pads have the following characteristics:... -
Page 60: Recycling Information
9.6 Recycling Information At the end of useful life, recycle the defibrillator and its accessories. Recycling Assistance For recycling assistance contact your local Defibtech distributor. Recycle in accordance with local and national regulations. Preparation For Recycling Items should be clean and contaminant-free prior to being recycled. -
Page 61: Electromagnetic Conformity
Electromagnetic Conformity 10.1 Guidance And Manufacturer’s Declaration The essential performance of the DDU-2000 Series AED is successful delivery of defibrillation therapy and accurate differentiation between shockable and nonshockable rhythms. DDU-2000 Series AEDs are intended for use within the electromagnetic environment specified below. The customer or the user of the DDU-2000 Series AED should assure that it is used within the stated environmental specifications. - Page 62 Electromagnetic Immunity (continued) IEC 60601 Compliance Electromagnetic environment – Immunity test test level level guidance Portable and mobile RF communications equipment should be used no closer to any part of the DDU-2000 Series AED, including 20 V/m cables, than necessary. The 80 MHz to recommended separation distance Radiated RF...
- Page 63 Regulatory Compliance Changes or modifications of this product, not expressly approved by Defibtech, may void the user’s authority to operate the equipment. This device complies with part 15 of the FCC Rules and Industry Canada Radio Standard RSS-210. Operation is...
-
Page 64: Glossary Of Symbols
Glossary Of Symbols Symbol Meaning High voltage present. SHOCK Button – Delivers defibrillation shock to the patient when the device is ready to shock. (Semi-Automatic units ONLY) SHOCK Required Indicator – Flashes to indicate that a shock is about to be delivered. (Fully-Automatic DDU-2200 ONLY) auto ON/OFF Button... - Page 65 Glossary Of Symbols (continued) Symbol Meaning Use by (yyyy-mm-dd). Defibrillation proof - Can withstand the effects of an externally applied defibrillation shock. Internally powered with defibrillator-proof BF-type patient applied parts (per EN 60601-1). Manufacturer. Y YY Y-MM-DD Y Y Y Y- MM -D D Y Y Y Y Y Y Y Y Date of manufacture.
-
Page 66: Contacts
Contacts Manufacturer Defibtech, L.L.C. 741 Boston Post Road, Suite 201 Guilford, CT 06437 USA Y Y Y Y- M M -D D Y Y Y Y- MM -D D Tel.: 1-(866) 333-4241 (Toll-free within North America) 1-(203) 453-4507 Fax: 1-(203) 453-6657 Email: sales@defibtech.com... - Page 67 Defibtech Automated External Defibrillator • Lifeline/ReviveR VIEW AUTO DDU-2200 • Lifeline/ReviveR VIEW DDU-2300 • Lifeline/ReviveR ECG DDU-2450 • Lifeline/ReviveR ECG+ DDU-2475 Operating Guide For concise guidance on set-up, use, maintenance and technical specifications DAC-U2530EN-AG rev E...
- Page 68 Setting Up the AED ................8 Notices Using the AED ...................10 Defibtech, L.L.C. shall not be liable for errors contained herein or for incidental or consequential Using the AED - ECG Display (DDU-2450 only) ..........damages in connection with the furnishing, performance, or use of this material. Information in this document is subject to change without notice.
-
Page 69: Quick Use Instructions
• Not breathing or not breathing normally Lifeline/ReviveR VIEW AUTO DDU-2200, Lifeline/ReviveR VIEW DDU-2300, Lifeline/ReviveR ECG DDU-2450, Lifeline/ReviveR ECG+ DDU-2475 AEDs may be used with Defi btech adult defi brillation pads (model number DDP-2001). For patients under 8 years old, or weighing less than 55 lbs (25 kg), use Defi btech child/infant defi brillation pads (model number DDP-2002), if available. -
Page 70: Diagram Of Components
DIAGRAM OF COMPONENTS Pads Connector Socket – Active Status Indicator (ASI) – Socket for pads connector Indicates the current status of the AED Battery Pack Battery Pack Opening ON/OFF Button – Eject Release Turns AED on and off Latch Softkey Buttons (Top, Center, Bottom) –... -
Page 71: Setting Up The Aed
SETTING UP THE AED The DDU-2000 Series AED is designed to be stored in a “ready” With the AED off, press state so that few steps are required to begin using the AED. AED status and release the CENTER Battery status softkey button. -
Page 72: Using The Aed
USING THE AED If the patient is unconscious or unresponsive, and is Note : When this Information Softkey Icon is present on the screen, not breathing or not breathing normally, ensure emergency the user may press the corresponding softkey button for additional information with video instruction. - Page 73 USING THE AED (continued) Note : When this Information Softkey Icon is present on the screen, the user may press the corresponding softkey button for additional information with video instruction. To exit, press the softkey button again. STAND CLEAR PERFORM CPR Follow instructions to perform CPR, if needed.
-
Page 74: Using The Aed - Ecg Display (Ddu-2450 Only)
USING THE AED - ECG DISPLAY THE DEFIBRILLATION PADS (DDU-2450 ONLY) HOW TO CONNECT THE PADS SELECTING ECG DISPLAY Insert the connector end of the defi brillation pad cable into the pads Mode of Operation The DDU-2450 allows the user to display connector socket on the top-left corner of the DDU-2000 Series AED as the patient ECG when the unit is being shown. -
Page 75: The Battery Pack
THE DEFIBTECH DATA CARD (OPTIONAL) HOW TO INSERT AND HOW TO INSERT AND REMOVE THE DEFIBTECH DATA CARD REMOVE THE BATTERY PACK The optional Defi btech Data Card (DDC card) is used to store event and audio information Before inserting the battery pack into the DDU- collected by the AED. -
Page 76: Checking Aed Status
CHECKING AED STATUS MAINTENANCE ROUTINE MAINTENANCE ACTIVE STATUS INDICATOR (ASI) The DDU-2000 Series AED is designed to be very low maintenance. Simple maintenance tasks The Active Status Indicator (ASI) should be visually checked on a regular basis to ensure that are recommended to be performed regularly to ensure its readiness (see sample maintenance the AED is ready for use. - Page 77 MAINTENANCE (continued) MAINTENANCE MODE (AED Maintenance) CLEANING AED Maintenence The AED Maintenance screen allows the user to select After each use, clean the DDU-2000 Series AED of any dirt or contaminants on the case and Rescue now such options as AED tests, software upgrades, data connector socket.
-
Page 78: Troubleshooting
TROUBLESHOOTING Symptom Possible Cause Corrective Action The following table lists the symptoms, the possible causes, and the possible corrective actions Make sure pads connector for common problems. Refer to the User Manual (available at www.defi btech.com) for detailed “ Pads missing” prompt Pads not connected to unit is oriented correctly and fully explanations on how to implement the corrective actions. -
Page 79: Warnings And Cautions
WARNINGS AND CAUTIONS WARNINGS (continued) WARNINGS (continued) • Some very low amplitude or low frequency VF rhythms may not • While in demonstration mode, the AED cannot perform a rescue. WARNINGS (continued) be interpreted as shockable. Some very low amplitude or low If at any time during the demonstration there is a need to perform •... -
Page 80: Technical Specifications
TECHNICAL SPECIFICATIONS (see User Manual for complete specifi cations) DEFIBRILLATION / MONITORING PADS OPERATING MODES MODEL SURFACE AREA TYPE AED MODE (ALL MODELS) ECG DISPLAY (DDU-2450 ONLY) Adult: DDP-2001 Adult: 12 inches (77cm Pre-connected, single-use, High resolution video display Displays ECG data and event information Child/Infant: DDP-2002 (nominal, each pad) non-polarized, disposable,... -
Page 81: Glossary Of Symbols
GLOSSARY OF SYMBOLS Symbol Meaning Symbol Meaning High voltage present. Meets the requirements of the European Medical Device Directive. SHOCK Button – Delivers defibrillation shock to the patient when the device is ready to shock. (DDU-2300/2450 ONLY) Meets the requirements of the Radio Equipment and Telecommunications Directive, 1999/5/EC. -
Page 82: Warranty Information
In the event whether purchased concurrently with the defibrillator as part of replacement, Defibtech shall have the right at its sole of a configuration or separately, shall be substantially free discretion to replace the item with a new, or refurbished, same For USA users only. -
Page 83: Contacts
CONTACTS Manufacturer Defibtech, L.L.C. 741 Boston Post Road, Suite 201 Guilford, CT 06437 USA Y Y Y Y Y Y Y Y Tel.: 1-(866) 333-4241 (Toll-free within North America) 1-(203) 453-4507 Fax: 1-(203) 453-6657 Email: sales@defibtech.com (Sales) reporting@defibtech.com (Medical Device Reporting) service@defibtech.com...
















Need help?
Do you have a question about the Lifeline/ReviveR ECG+ DDU-2475 and is the answer not in the manual?
Questions and answers