Table of Contents
Advertisement
Available languages
Available languages
Quick Links
Advertisement
Table of Contents

Summary of Contents for Repose Ultracore
- Page 1 instructions for use www.reposedirect.com...
-
Page 2: Product Specification
PRODUCT SPECIFICATION Product Foam Density / Hardness Dimensions (cm) Support Walls Width Height Length Surface Repose® ultracore® 33 / 120 39 / 200 Repose ultracore plus 33 / 160 39 / 200... -
Page 3: Product Description
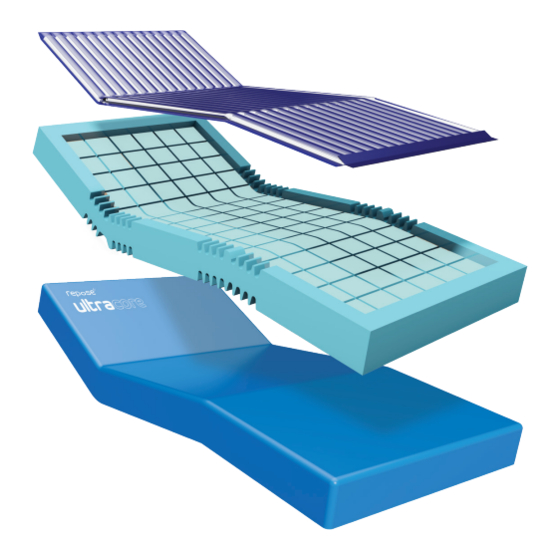
Repose ultracore combines several different components that when combined, provide healthcare professionals and patients exceptional levels of comfort and pressure redistribution properties. At its core, is the Repose mattress overlay, which has over 18 years of clinical evidence to support its ease of use and durability. -
Page 4: Product Labelling
The mattress should be unrolled within 21 days of receipt. Once unrolled open the PATIENT OFF THE PRODUCT mattress cover and place the inflatable Repose ultracore inner overlay, clear side up inside the KEEP AWAY FROM SHARP OBJECTS AND DIRECT HEAT foam surround. - Page 5 Do not store mattresses next to radiators or other heating devices • It is recommended that the ultracore is handled by a minimum of two people. Do not drag! • Do not place heavy objects on the mattress surface when not in use •...
- Page 6 CARE & MAINTENANCE FOR REPOSE INNER Repose® Ultracore® mattress is designed for the prevention and treatment of pressure ulcers and is intended to be used in conjunction with an appropriately sized bed frame, as part of an overall pressure ulcer prevention program.
- Page 7 Inspect the inner and outer surfaces of covers and their zip fasteners regularly for signs of damage. If the cover is stained, soiled or torn, the foam and Repose core should be examined. Damaged / soiled covers and mattresses should be reported to the ward / department manager.
-
Page 8: Cpr Mode
The interior foams meet the requirements of The Furniture & Furnishings (Fire) (Safety) Regulations 1988. UK specification ultracore mattress meets the requirements of BS 7177: 2008 for medium hazards European specification ultracore mattress meets the requirements of BS EN 597-1: 1995 / BS EN 597-2: 1995... -
Page 9: Warranty
WARRANTY Repose ultracore warranty is valid from time of shipping. If a defect or fault is discovered, please contact Repose customer service on Tel: 0044 (0) 1495 235800 / email: info@reposedirect.com immediately. Frontier Medical Group will not accept responsibility for damage caused by misuse or non-observance of the instructions set out in this instruction for use document. - Page 10 PRODUCTSPECIFICATIE Artikel Schuimdensiteit/hardheid Afmetingen (cm) Ligoppervlak Boord Breedte Hoogte Lengte Repose® ultracore® 33 / 120 39 / 200 Repose ultracore plus 33 / 160 39 / 200...
- Page 11 Repose ultracore combineert verschillende elementen die, wanneer ze worden samen gebracht, aan gezondheidsmedewerkers en patiënten een uitzonderlijk niveau van comfort en drukverdeling bieden. In de kern van de matras bevindt zich de Repose ultracore binnenmatras, een systeem met meer dan 18 jaar klinische evidentie als bewijs van zijn gebruiksvriendelijkheid en degelijkheid.
-
Page 12: Product Label
CARE & MAINTENANCE FOR REPOSE INNER Repose® Ultracore® mattress is designed for the prevention and treatment of pressure ulcers and is intended to be used in conjunction with an appropriately sized bed frame, as part of an overall pressure ulcer prevention program. - Page 13 Verwijder voorzichtig de verpakking opdat de hoes van de matras niet wordt beschadigd. Repose ultracore kan reeds worden gebruikt één uur na het ontrollen van de matras. Het is echter mogelijk dat het meer tijd in beslag neemt vooraleer de matras zijn oorspronkelijke vorm terug heeft aangenomen.
- Page 14 CARE & MAINTENANCE FOR REPOSE INNER Repose® Ultracore® mattress is designed for the prevention and treatment of pressure ulcers and is intended to be used in conjunction with an appropriately sized bed frame, as part of an overall pressure ulcer prevention program.
- Page 15 WAARSCHUWING: Inspecteer regelmatig de binnen- en buitenzijde van de hoes en de ritssluiting op schade. Indien de hoes gevlekt is, bevuild of gescheurd moeten het schuim en de Repose Ultracore binnenmatras worden geïnspecteerd. Beschadigde/bevuilde hoezen en matrassen moeten worden gerapporteerd aan de dienst/departementsverantwoordelijke. Indien de binnenkant van de matras vochtig of erg bevlekt is, moet de matras uit dienst genomen worden.
- Page 16 Het schuim voldoet aan de vereisten van Furniture & Furnishing (Fire) (Safety) Regulations 1988 Repose Ultracore matras (UK specificatie) voldoet aan de vereisten van BS 7177: 2008 voor middelgrote gevaren Repose Ultracore matras (Europese specificatie) voldoet aan de vereisten van BS EN...
-
Page 17: Garantie
GARANTIE De garantie op de Repose Ultracore matras is geldig vanaf het moment van verzending. Indien een defect of fout wordt vastgesteld, neem onmiddelijk contact op met Repose klantendienst op Tel: 0044 (0) 01495235800 / e-mail: info@reposedirect.com. Frontier Medical Group neemt geen verantwoordelijkheid voor schade veroorzaakt door oneigenlijk gebruik of door het niet naleven van de instructies in de gebruikershandleiding. - Page 20 Tel: +1 800 3030 9544 Email: info@frontier-group.co.uk Email: infoger@frontier-group.co.uk Email: contactus@therapeutics.com www.frontier-medical-germany.de www.frontier-therapeutics.com www.frontier-group.co.uk Repose is covered by UK patent no. 2328152 Ultracore is a registered trade mark of Frontier Medical Group. © December 2020 Frontier Therapeutics Limited. All rights reserved. GP0052_B93_08...










Need help?
Do you have a question about the Ultracore and is the answer not in the manual?
Questions and answers