Summary of Contents for GERATHERM Spirostik
- Page 1 Spirostik Serial numbers: xx|8|001|yyy and 2001xxxxx Instructions for Use Version: 6 Release date: 14. July 2021 Please read carefully and store in a place which is always accessible for future consultation!
- Page 2 Language: English Affected serial numbers: xx|8|001|yyy and 2001xxxxx Software version: valid from 1.0.1 Geratherm® Respiratory GmbH Kasernenstraße 4 97688 Bad Kissingen, GERMANY Tel.: +49 971 7857043-0 Fax: +49 971 7857043-30 info@geratherm-respiratory.com 0494 www.geratherm-respiratory.com EUDAMED SRN: © Geratherm® Respiratory GmbH DE-MF-000006818...
-
Page 3: Table Of Contents
Consumable Items / Auxiliary Materials ......... 25 Safety in Handling ..............27 General Safety at Work and Personnel Qualification ..... 28 The Technical State of Spirostik and System Construction ... 29 Operation / Servicing and Maintenance ........31 Electromagnetic Compatibility (EMC) ..........32 Cleaning and Disinfection ............. - Page 4 Technical Specifications ............62 12.1 Technical Data ................62 12.2 Installation and Operating Conditions ........... 64 12.3 Electrical Safety Concept .............. 65 12.3.1 Spirostik with Medical Device Cart and Isolation Transformer ..65 Page 4 Version: 6 | Release date: 14. July 2021...
- Page 5 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 12.3.2 Spirostik without Medical Device Cart and without Isolation Transformer .................. 66 12.4 Electromagnetic Compatibility / EMC Guidelines ......67 12.4.1 Emitted Interference Guideline and Manufacturer Declaration ..67 12.4.2 Interference Resistance for all ME Systems Guideline and Manufacturer Declaration .............
-
Page 6: General Information
This IFU is a component of the product in accordance with DIN EN ISO 60601-1. It should make it easier to familiarise yourself with Spirostik, as well as give you instructions about its intended use and safe operation. This IFU has been written for healthcare professionals who are qualified to perform spirometric examinations. -
Page 7: Abbreviations
If, in spite of careful reading of this IFU, you require more information, please contact your specialist retail partner on site. You can obtain the contact details via a form provided by the manufacturer at www.geratherm-respiratory.com/login/. Abbreviations The following simplified style of writing and abbreviations are used hereinafter to make this IFU easier to read. - Page 8 Not observing and not avoiding the situation may lead to minor or moderate injuries. Not observing this warning information may lead to faults or malfunctions of the Spirostik or may indicate that something in Attention its environment may be damaged.
-
Page 9: Symbols
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Symbols Symbols displayed in this IFU, on the medical device itself and / or on its packaging are standardised symbols. Symbol Explanation Follow the instructions for use! Applied part from type BF corresponding to... - Page 10 Serial no.: xx|8|001|yyy and 2001xxxxx Symbol Explanation For single use only! This symbol does not refer to the Spirostik itself, but to the consumable items used in connection with it. This is applied to the respective packaging and must be observed.
- Page 11 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Symbol Explanation Fragile, handle with care The package contains a product that must be handled with appropriate care to prevent damage during transport and storage. Keep away from rain The package contains a product that must be protected from moisture during transport and storage.
-
Page 12: Copyright
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Copyright The manufacturer reserves all rights to this document and the information contained therein. No part of this document or the information contained herein may be reproduced or transmitted without the written consent of the manufacturer. All information or brand names of a third party contained in this document are subject to the copyright of that third party. -
Page 13: Conditions Of Use
Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Conditions of Use Any other use of Spirostik which is not described in this IFU is deemed improper use. The responsible organisation of Spirostik alone is liable for any direct or indirect damage resulting from not adhering to these conditions. They are then... -
Page 14: Indication
2.1.1 Indication With Spirostik, pulmonary function examinations can be carried out for diagnosing, monitoring of process, screening and assessing the severity of pulmonary diseases. In particular, this includes: Obstructive diseases •... -
Page 15: Contraindication And Side Effect
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 2.1.2 Contraindication and Side Effect 2.1.2.1 Contraindications The following contraindications apply for spirometric examinations: Contraindications Absolute Relative Patients with a new (< 1 month old) myocardial infarction Aneurysm of the ascending aorta... -
Page 16: Side Effects
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 2.1.2.2 Side Effects If there are no contraindications and the examination is carried out in accordance with the descriptions in this IFU, side effects rarely occur in pulmonary function examinations. These can be... -
Page 17: Definition Of The Groups Of People
Spirostik on the patient after verifiable instruction by the responsible organisation and / or is responsible for rectifying faults to the Spirostik, as well as its calibration. Users must be aware of the clinical meaning and, for example, be a physician, physician’s assistant, assistant or trained... -
Page 18: Intended Use
11.1 "Expected Service Life") and the maintenance work required for this (see chap. 8 "Servicing / Maintenance"). The Spirostik may only be used for the duration of its service life if the specifications are observed. Any changes to Spirostik, in particular unauthorised modifications, are prohibited. -
Page 19: Original Spare Parts / Accessories / Optional Expansions
The installation or use of other products can, under certain circumstances, negatively change constructive prescribed properties of Spirostik and, in the worst case, impair the safety of the patient, user / operator and / or third parties. The manufacturer assumes no liability for such consequences. -
Page 20: Original Spare Parts / Accessories
Ambistik IFU Component Description / name Supply scope in units Spirostik, Case 177882 For safe and secure transport of Spirostik. For organised and structured storage of components and accessories. Page 20 Version: 6 | Release date: 14. July 2021... - Page 21 340968 [old:10.401] Single use disposable flow sensor with low deadspace and Softclip noseclip for use with the Spirostik and Spirostik Complete. Sensor codes validated during production ensure measurement quality and avoid frequent calibration. Version: 6 | Release date: 14. July 2021...
- Page 22 USB-Extension Cable, 30 cm 583407 30 cm extension cable to [old:10.837] connect USB based devices to PC. Quick Start Guide, Spirostik 531666 [old:40.001-02] Short instruction manual for quick start of Spirostik. Page 22 Version: 6 | Release date: 14. July 2021...
-
Page 23: Optional Expansions
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 2.2.1.2 Optional Expansions The following add-ons can be purchased via specialist retail partners. You will find: • A list of specialist retail partners as an insert in this IFU or in your medical device book, as well as the most updated version at www.geratherm-respiratory.com/login/... - Page 24 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Component Description / name Provocation 403680 [old:10.507] For performing bronchial provocation tests with BLUE CHERRY® diagnostic platform. Offers pre-defined and user configurable protocols with timers for testing and dosage administration.
-
Page 25: Consumable Items / Auxiliary Materials
Consumable Items / Auxiliary Materials The following items were tested by the manufacturer for Spirostik. The use of other consumable items as well as auxiliary materials with different properties is deemed improper use. You will find a list of specialist retail partners as an insert in this... - Page 26 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Component Description / name For wipe disinfection, alcoholic quick-acting Bacillol Tissues (BODE Chemie GmbH) SprayIn (Dr. Deppe GmbH) For disinfection bath depend- with low chloride concentration Disinfectant ing on ...
-
Page 27: Safety In Handling
In spite of this, dangers of injury to users / operators, patients and third parties as well as damage to Spirostik or other materials may occur if this is: •... -
Page 28: General Safety At Work And Personnel Qualification
Always comply with general national regulations on accident prevention! Instruct users / operators accordingly! • Safety and warning information on Spirostik may not be altered or removed! Have missing or not readable information replaced immediately! • When working with auxiliary materials, always... -
Page 29: The Technical State Of Spirostik And System Construction
Misdiagnosis caused by measurement error. Therefore: • Do not overstress Spirostik! Use with care! • Do not modify or use Spirostik or total system contrary to the respective manufacturer's specification! • Only use Spirostik within its expected service life, determined by the manufacturer! •... - Page 30 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx • Ensure that Spirostik cannot fall down! Otherwise, the functionality must be checked properly before putting into operation! Reason: Electric shock and / or misdiagnosis due to loss of electrical safety caused by exceeding the recommended maintenance schedule.
-
Page 31: Operation / Servicing And Maintenance
Therefore: • In principle, observe chap. 12 “Technical Specifications”! • Do not operate Spirostik if there are flammable or explosive gases in the room! • Do not operate Spirostik near the magnetic field of an MRT system! Possible danger to life. -
Page 32: Electromagnetic Compatibility (Emc)
Therefore: • While using Spirostik, do not use any transmitting devices (e.g. mobile phones, portable phones, power lines …) within close proximity (< 30 cm) that exceed the immunity levels as specified in the EMC guidelines! •... -
Page 33: Cleaning And Disinfection
Possible danger to life. Reason: Cross-contamination. For this: Observe the general medical principles! Therefore: • Clean and disinfect the Spirostik and its reusable components as instructed by the manufacturer at regular intervals as specified! Possible severe physical injury. Reason: Contamination with transferable germs during improper disposal. -
Page 34: Structure And General Function Of Spirostik
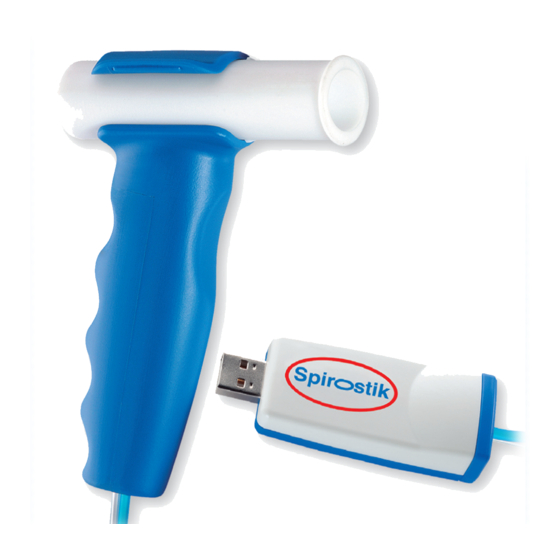
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Structure and General Function of Spirostik Hardware 4.1.1 Overview Easy to use snap-in handle Spirostik (see also chap. 7.6) Spiraflow flow sensor (see also chap. 4.1.2.1) Type label / (on the bottom of the device) (see also chap. 14) USB-extension cable, 30 cm (see also chap. -
Page 35: Connectors / Interfaces Of The Spirostik
Spirostik. The Spirostik connects directly with the PC and forms a medical electrical system (ME system) with the computer where the application software is installed. The following instructions are intended to safely form such a system, considering the electrical safety concept of the Spirostik (see chap. - Page 36 Independent of this, the following should also be observed: Possible danger to life. Reason: Electric shock. Misdiagnosis. Therefore: • Do not modify or use Spirostik or total system contrary to the respective manufacturer's specification! • Under no circumstances use or connect any...
- Page 37 (interfaces, plugs, etc.) that are not isolated from the mains with an isolation voltage of 4 kV! • When connecting Spirostik to other devices, make sure that all connections and cables are secured and cannot come loose by themselves! •...
-
Page 38: Bluetooth / Computer / Printer Data Connection
Any printer compatible with Windows can be used for this. 4.1.3.2 Power Supply The Spirostik is powered directly via the 5 V of the USB interface. Technical Protection Measures Spirostik has been designed and built in accordance with the recognised state of technology and the requirements of the applicable, safety-relevant regulations. -
Page 39: Software
Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Software The measurement device (Spirostik) is supplied with BLUE CHERRY® software. This serves to manage patient and examination data as well as carry out, depict, process and record measurements with the devices of the manufacturer. -
Page 40: Transport, Storage And Assembly
Transport, Storage and Assembly Transport to the Location of Use Spirostik is secured against damage during transport in a case. The packaging must be kept by the user / operator in case the device must be returned to the authorised specialist retail partner or manufacturer. -
Page 41: Assembly
As a consumable, the flow sensor must be replaced constantly, which is therefore not considered assembly. The Spirostik is connected directly to the PC via the USB interface [1]. Alternatively, the USB extension cable [2] can be placed between the PC and the Spirostik, increasing the range of the Spirostik by a further 30 cm. -
Page 42: Operation
Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Operation The initial operation of Spirostik, as well as any return to operation after service work in accordance with the duties of the responsible organisation (see chap. 8.2 “Servicing / Maintenance by the User / Operator”), may be carried out by the responsible organisation's own authorised specialist personnel. -
Page 43: Returning To Operation After Servicing / Cleaning Work
Servicing work here also includes the necessary checks which are necessary if Spirostik should fall down. Before the Spirostik can be used for measurements again, all components must be properly connected / mounted and calibrated. Calibration also fulfils the regulatory requirement of MPBetreibV §7 for functional testing after maintenance work. -
Page 44: Operating Instructions
Reason: Penetrating liquid. Uncleanliness. External exposure. Therefore: • Do not use any liquids near the Spirostik! • Do not expose Spirostik or the entire system to dust or Attention other contamination! • Do not drop any objects on Spirostik! Do not lay any... -
Page 45: Preparing The Device For Operation
Chap. 6.1.1ff “Calibrate Spirostik / Set up funtional Operations” Switching Spirostik On / Off As soon as the Spirostik is connected to the PC by the USB interface, the device is automatically detected and started. It is ready for operation. -
Page 46: Inserting The Flow Sensor
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Inserting the Flow Sensor 1. Insert Spiraflow [2] into the handle [1] For this: Align the Spiraflow so that the interrupted guide rib [2a] and the nose on the handle are on the... -
Page 47: Calibrating Spirostik
Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Calibrating Spirostik There are various processes to be carried out for Spirostik in order to calibrate or validate the volume measurement. Insert the flow sensor as described in chap. 7.4 "Inserting the Flow Sensor". Connect the calibration pump to the flow sensor (on the mouthpiece). -
Page 48: Using Spirostik / Carrying Out Measurements
Therefore: • While using Spirostik, do not use any transmitting devices (e.g. mobile phones, portable phones, power lines …) within close proximity (< 30 cm) that exceed the immunity levels as specified in the EMC guidelines! •... -
Page 49: Removing The Flow Sensor
Observe the general medical principles! Therefore: • Do not use single use products more than once! • Clean and disinfect Spirostik and its reusable components as instructed by the manufacturer at regular intervals as specified! Possible severe physical injury. Reason: Contamination with transferable germs during improper disposal. - Page 50 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Proceed as follows for removal: 1. Unlock the handle For this: Slightly bend up the locking device [1] by pressure from below. 2. Remove the flow sensor [2] from the handle (towards mouthpiece side) 3.
-
Page 51: Servicing / Maintenance
The expected service life of Spirostik is 8 years. In its development, a great deal of value was placed on making the servicing of all device components as simple as possible. -
Page 52: Servicing / Maintenance By The User / Operator
“Servicing / Maintenance by the User / Operator”! Attention Servicing / Maintenance by the User / Operator In order to ensure a flawless operation of Spirostik over its whole service life, regular servicing and repairs, if applicable, are required. Interval... -
Page 53: Checking For Damage
8.2.1 Checking for Damage 8.2.1.1 General All parts of Spirostik should be checked for visible mechanical damage (cracks, tears) each day. If damage is ascertained, the corresponding component must be replaced. For replacing components see: •... -
Page 54: Checking / Replacing The Handle
3. Slide the blue tube onto the connection piece with the marking (blue, slightly highlighted ring [3]). When sliding the blue tube onto the Spirostik, make sure that the connection is correct! Page 54 Version: 6 | Release date: 14. July 2021... -
Page 55: Cleaning And Disinfection
The devices of the manufacturer were designed in such a way that minimal effort is required for cleaning and disinfection. This is why just a few tasks are necessary to keep Spirostik functional and clean. The following intervals apply: Component... -
Page 56: Disinfection
Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Disinfection All parts of the Spirostik can be cleaned of dirt with a soft cloth using a cleaning solution / weak soapy water. All parts of Spirostik which come into contact, or could come into contact, with the patient must be treated with a surface disinfection. - Page 57 Follow the instructions on the concentration and dwell time stated by the cleanser and disinfectant manufacturer! • Do not place Spirostik in the cleaning and disinfection solutions! The device contains electrical components that will be damaged by doing so! Reason: Avoid penetration of liquid into electrical components.
- Page 58 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx When selecting a suitable disinfectant, please observe: • Chap. 2.2.2 “Consumable Items / Auxiliary Materials” For selection of suitable disinfectants for surface / wipe disinfection also note: • The separate IFU Cleaning and Disinfection...
-
Page 59: Fault Indication And Repair
Serial no.: xx|8|001|yyy and 2001xxxxx Fault Indication and Repair Simple errors which occur when using Spirostik can be recognised quickly and rectified using the following table. If you cannot find the error in the table or the problem cannot be rectified using the method described, please contact your specialist retail partner. -
Page 60: Decommissioning / Disposal
11.3.2 Spirostik Spirostik is an active medical device and is thus subject to the WEEE directive 2012 / 19 / EU and the German law on electrical and electronic devices (ElektroG) for the disposal for old electrical items. -
Page 61: Infectious / Contaminated Single Use Items
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 11.3.3 Infectious / Contaminated Single Use Items All contaminated items such as single use flow sensors, filters, mouthpieces and noseclips must be disposed of via hospital or medical practice waste. Possible severe physical injuries. -
Page 62: Technical Specifications
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Technical Specifications 12.1 Technical Data Medical device: Class IIa (in accordance with MDD 93 / 42 Council Directive of 14.6.1993 annex IX) Dimensions: (L) 76.5 mm x (W) 30 mm x (H) 18.5 mm... - Page 63 Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Volume: Measuring range: 0 – 20 l Accuracy: ±3 % oder 50 ml Minimum PC system requirements: Standard: at least EN 62368-1 / EN 60950 recommended EN 60601 Processor: X86 / amd64 compatible,...
-
Page 64: Installation And Operating Conditions
12.2 Installation and Operating Conditions The following conditions supplement chap. 2.2 "Intended Use" and must be observed to maintain the properties of the Spirostik guaranteed by the manufacturer and for the safety of the patient and operator. Storage / Transport: min. -
Page 65: Electrical Safety Concept
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 12.3 Electrical Safety Concept 12.3.1 Spirostik with Medical Device Cart and Isolation Transformer Version: 6 | Release date: 14. July 2021 Page 65... -
Page 66: Spirostik Without Medical Device Cart And Without Isolation Transformer
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 12.3.2 Spirostik without Medical Device Cart and without Isolation Transformer Page 66 Version: 6 | Release date: 14. July 2021... -
Page 67: Electromagnetic Compatibility / Emc Guidelines
12.4.1 Emitted Interference Guideline and Manufacturer Declaration Guidelines and manufacturer’s declaration – electromagnetic emissions The Spirostik is determined for operation in an electromagnetic environment as specified below. The user / operator of the Spirostik should ensure that it is operated in this environment. Measurement of Compliance Electromagnetic environment –... -
Page 68: Interference Resistance For All Me Systems Guideline And Manufacturer Declaration
Guidelines and manufacturer’s declaration – electromagnetic interference immunity The Spirostik is determined for operation in an electromagnetic environment as specified below. The user / operator of the Spirostik should ensure that it is operated in this environment. Measurement of IEC 60601 – test... -
Page 69: Interference Resistance For Non-Life-Supporting Me Systems Guideline And Manufacturer Declaration
Guidelines and manufacturer’s declaration – electromagnetic interference immunity The Spirostik is determined for operation in an electromagnetic environment as specified below. The user / operator of the Spirostik should ensure that it is operated in this environment. Measurement of IEC 60601 -level... - Page 70 If the measured field strength at the site where the Spirostik is used exceeds the abovementioned compliance levels, the Spirostik should be observed to demonstrate its intended function.
-
Page 71: Recommended Safety Distances For Non-Life-Supporting Me Systems
Recommended safety distance between portable and mobile RF communication devices and the Spirostik The Spirostik is determined for operation in an electromagnetic environment in which the RF disturbances are controlled. The user / operator of the Spirostik may help to avoid... -
Page 72: Safety Of Product And Material
DIN EN ISO 10993- 1:2017-04 "Biological evaluation of medical devices" (biocompatibility). Spirostik is a class IIa active medical device. Conformity with the underlying standards and directives is certified in the declaration of conformity which is included in the documentation accompanying the device. -
Page 73: Product Labeling / Type Label
93 / 42 / EEC with identification of the involved notified body [12] Date of manufacture Safety symbols have been applied to the Spirostik type label. It may not be changed or removed. If information should become unreadable, the type label should be replaced immediately. -
Page 74: Warranty And Service
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Warranty and Service 15.1 General Conditions The manufacturer guarantees that the products you have purchased fulfill the listed technical data and that the medical devices are free from technical material and production defects. -
Page 75: Packaging And Shipping
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx 15.3 Packaging and Shipping To avoid damage during transport, devices must be sent along with the warranty claim in the original packaging. This also applies for defective devices being repaired. Transport damage arising from improper packing is the responsibility of the customer. -
Page 76: Authorised Specialist Retail Partner
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Authorised Specialist Retail Partner You can reach your responsible specialist retail partner via a contact form of the manufacturer www.geratherm-respiratory.com/login/ See depositors Page 76 Version: 6 | Release date: 14. July 2021... -
Page 77: Attachment - Declaration Of Conformity
Instructions for Use Spirostik Serial no.: xx|8|001|yyy and 2001xxxxx Attachment – Declaration of Conformity The Spirostik declaration of conformity is enclosed with each device by the manufacturer. Version: 6 | Release date: 14. July 2021 Page 77...
















Need help?
Do you have a question about the Spirostik and is the answer not in the manual?
Questions and answers