GERATHERM Spirostik User Manual
Hide thumbs
Also See for Spirostik:
- Instructions for use manual (77 pages) ,
- Instructions for use manual (94 pages)
Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for GERATHERM Spirostik
- Page 1 Spirostik User Manual Version 1.2.2 Date 24th October, 2014...
-
Page 2: Table Of Contents
Norms and Guidelines ............7 2.2.1.2 Electromagnetic Compatibility ..........7 2.2.2 Safe Use of Spirostik .............. 8 2.2.2.1 Connecting Spirostik, double hose and handle ..... 9 2.2.2.2 Replace handle ..............10 2.2.2.3 Connecting the Flow sensor into the handle ...... 11 2.2.2.4 Removing the flow sensor from the handle ....... -
Page 3: Welcome To Blue Cherry
We strongly recommend reading this manual carefully in order to avoid incorrect use or damage to the device. Geratherm Respiratory does not take responsibility for any direct or indirect damage to the Spirostik, if it is not operated in accordance with this manual. Users must observe precautions, warnings and instructions. -
Page 4: Spirostik
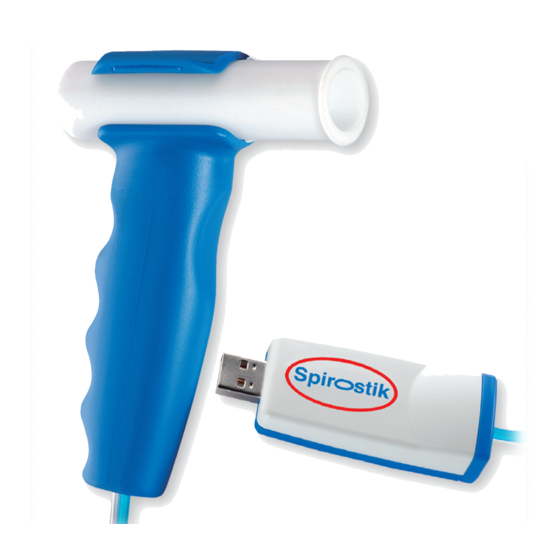
Description and Scope of Delivery 2.1.2 Description Spirostik is a PC based spirometry system. It is used in pulmonary function testing to determine multiple parameters in spirometry. Spirostik consists of a USB electronic module including pressure transducers, flow sensor and a 2m double tube connecting the pressure transducer and the flow sensor. -
Page 5: Scope Of Delivery
Blue Cherry has a unique report interface allowing the preview and print of chosen numeric and Graphic data and the editing of curves retrospectively. 2.1.3 Scope of Delivery Spirostik includes the following components: Component Name and Description USB Spirostik Electronic module connecting to USB port... -
Page 6: Safety Instructions
- the device is used in accordance with this manual - user observes all technical instructions - the electrical installation where the Spirostik is used fulfil the requirements of VDE 0100 Warranty will be void if improper accessories or consumables are used. -
Page 7: Norms And Guidelines
The Geratherm Respiratory products are manufactured in accordance with DIN EN ISO 13485. According to the European directive 93/42/EEC, Spirostik fulfils the requirements of annex II. In addition the following norms are fulfilled: - DIN EN 60601-1 (Medical electrical device) -
Page 8: Safe Use Of Spirostik
Spirostik 2.2.2 Safe Use of Spirostik Spirostik is part of a PC based medical device. It is highly recommend that the user observes all warning notices in order to ensure a safe use of the device. The Spirostik complete should not be used in conjunction with multiple connection plug sockets or extension leads. -
Page 9: Connecting Spirostik, Double Hose And Handle
The double hose should be firmly connected with the Spirostik, with the blue hose being connected to the connection marked dark blue. Always ensure the double hose is not bent or damaged. -
Page 10: Replace Handle
Make sure to connect the blue hose (1) to the right top (blue marked connector) and the transparent hose (2) to the left bottom connector. In order to secure a save use of the Spirostik device, make sure that the tubing is connected properly as shown in the picture below: SPIROSTIK_MAN_ENG_V1.2.2_REV01.DOCX... -
Page 11: Connecting The Flow Sensor Into The Handle
Spirostik 2.2.2.3 Connecting the Flow sensor into the handle In order to ensure the safe function of the Spirostik, the flow sensor must be connected correctly to the handle. Please read the following procedure carefully. Place the flow sensor in the ‘mouth’ of the grab handle, ensure the... -
Page 12: Removing The Flow Sensor From The Handle
User Manual Spirostik 2.2.2.4 Removing the flow sensor from the handle In order to protect the user from cross contamination, the flow sensor can be removed from the handle with a single hand without touching the used flow sensor. Please read the following procedure... - Page 13 User Manual Spirostik Gently pushing the upper part of the handle with the thumb opens the ‘mouth’ of the handle, so that the flow sensor can fall out. This procedure can be done directly over the waste facility. SPIROSTIK_MAN_ENG_V1.2.2_REV01.DOCX Page 13...
-
Page 14: Combination With Other Devices
User Manual Spirostik 2.2.3 Combination with other devices Connecting additional USB devices and high processor load can reduce the sample rate and therefore cause an error. We strongly recommend not connecting additional USB devices except mouse, keyboard and printer and not installing additional software. - Page 15 User Manual Spirostik Situation Medical used room Non-medical used Possible room solution Within patient Out of area patient area resource A and B within patient area IEC 60601 IEC 60601 resource A and B for B: within patient area additional...
-
Page 16: Disposal
The first 2 digits define the manufacturing year. The example 13 shows the device was manufactured in 2013. The identifier |8|001| is a product specific code. The remaining digits define the device number of the Spirostik. REF: designates the part number for the Spirostik 40 001. SPIROSTIK_MAN_ENG_V1.2.2_REV01.DOCX Page 16... -
Page 17: Used Symbols
93/42 ECC for medical devices and fulfil the requirements of annex II 0494 of the directive Maintenance The Spirostik has been designed to ensure it is a low maintenance device. Only minor maintenance is required to ensure trouble free operation. Interval Maintenance work After every patient resp. -
Page 18: Warranty
Geratherm Respiratory, and respectively equal to installation date if purchased from a sales partner. All service must be provided by Geratherm Respiratory or an authorized service partner of Geratherm Respiratory. Geratherm Respiratory will not accept warranty claims on any unauthorised repairs. -
Page 19: Technical Data Spirostik
User Manual Spirostik 4. Technical Data Spirostik Modifications are not permitted with this device Technical Data: Dimensions 76,5mm x 30mm x 18,5mm (L x W x H) Weight Electrical Data: IP protection type: IPX0 IEC 529 Protection class: Classification according to...















Need help?
Do you have a question about the Spirostik and is the answer not in the manual?
Questions and answers