Lifelines trackit T4A User Manual
Eeg amplifier
Hide thumbs
Also See for trackit T4A:
- How-to manual (14 pages) ,
- User manual (67 pages) ,
- User manual (69 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Lifelines trackit T4A
- Page 1 USER MANUAL Imagine EEG Anywhere...
- Page 2 CCN: CCN119 Digitally signed by Geoff Salter Created Date: 2019.10.14 08:56:20 +01'00' Digitally signed by Michael Hulin Checked Date: 2019.10.15 15:29:51 +01'00' Digitally signed by David Hulin DN: cn=David Hulin, o=Lifelines Ltd, Approved ou=Development, email=david.hulin@llines.com, c=GB Date: 2019.10.15 15:54:15 +01'00'...
- Page 3 The owner of this system has the sole responsibility for any malfunction resulting from improper use or maintenance, or repair done by anyone other than a qualified Lifelines Neuro representative and for any malfunctions caused by any parts that have been damaged or modified by anyone other than a qualified Lifelines Neuro representative.
-
Page 4: Disclaimers & Warranties
The information in this section is subject to change without notice. Except as stated below, Lifelines Ltd makes no warranty of any kind with regard to this material, including, but not limited to, the implied warranties of merchantability and fitness for a particular purpose. Lifelines shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance or use of this material. - Page 5 The manufacturer and distributor consider themselves responsible for the equipment’s safety, reliability and performance only if: y any peripheral equipment to be used with the Trackit T4A system is supplied by third-party providers recommended by the manufacturer; y assembly operations, extensions, readjustments, modifications, or repairs are carried out by persons authorised by the manufacturer;...
-
Page 6: Table Of Contents
Overview ........................................... 20 3.2 Laptop installation and operation ..............................21 3.3 Fitting the battery packs ..................................22 3.4 Connecting the Trackit T4A Amplifier ............................23 3.5 Switching the Amplifier on and off .............................. 25 3.6 Bluetooth ...........................................27 3.7 SD Card ..........................................29 3.8 The T4A Bag ........................................ - Page 7 Appendix 7: Manufacturer’s Declaration ........................48 Illustrations Figure 1: Connecting the Trackit T4A Amplifier – Clinical Use ...................20 Figure 2: Connecting the Trackit T4A Amplifier - Home Use ....................21 Figure 3: Battery replacement ..................................22 Figure 4: Battery capacity display ................................22 Figure 5: Connecting the Trackit T4A Amplifier (PCU/front face) ..................23...
-
Page 8: Overview And Technical Description
General description Indications for use The Trackit T4A EEG Amplifier is intended to be used as a front-end amplifier to acquire, store and transmit electrophysiological signals (wireless or cabled). CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. -
Page 9: Warnings And Cautions
Do not use the Trackit T4A EEG Amplifier in an MRI environment, in an oxygen rich environment or during defibrillation This equipment is intended to be used by a healthcare professional and in accordance with these instructions for use which must be read in their entirety before the device is used. - Page 10 Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Trackit T4A, including cables specified by Lifelines Ltd. Otherwise, degradation of the performance of this equipment could result.
-
Page 11: Explanation Of Symbols
USER MANUAL Overview and Technical Description Explanation of symbols Meaning Symbol Symbol Meaning Follow operating instructions Type BF applied part Input connection Input/output connection Bluetooth Special recycling required* Memory card read/write Consult warnings in User Manual Remote event pushbutton On/Off and patient event switch Battery door access Manufacturer –... -
Page 12: The Amplifier And Its Parts
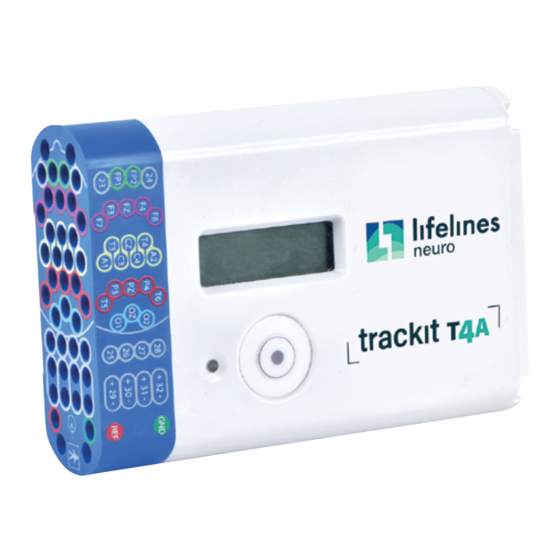
Overview and Technical Description The Amplifier and its parts The Trackit T4A EEG Amplifier is a 32-channel electroencephalograph recorder intended to acquire EEG signals in an ambulatory environment. It is powered by either one or two lithium-polymer battery packs and is fitted with an internal, non-replaceable, lithium-ion backup battery. -
Page 13: Specifications And Safety
MANUAL Overview and Technical Description Specifications and safety Refer to Appendix 1 for specifications. The Trackit T4A amplifier has been certified and complies with the following standards: Standard Description IEC 60601-1 and Standard for medical electrical equipment, general requirements and particular IEC 60601-2-26 requirements for EEG systems. -
Page 14: Description Of The Components
Description of the components The Trackit T4A Amplifier The Trackit T4A amplifier provides 32 channels (28 referential, 4 bipolar) with built-in calibration and electrode impedance measurement. The amplifier has built in type-BF patient isolation and has a USB interface to the PC. - Page 15 SD Memory Card The SD card is used to store the EEG data recorded by Trackit T4A. Storage cards of varying capacity are available in the SD format. The Trackit T4A supports SD cards with a capacity up to 32GB with a FAT32 file system.
-
Page 16: Replaceable Parts
View on-going EEG traces. Replaceable parts Lifelines Ltd. will make available on request circuit diagrams, component part lists, descriptions, calibration instructions, or other information that will assist service personnel to repair those parts that are designated by Lifelines Ltd. as repairable by service personnel. -
Page 17: Installation And Maintenance
USER MANUAL Installation and Maintenance Installation and Maintenance The following section must be read and understood before the equipment is switched ON. Medical electrical equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in the Appendix. The function or safety of the equipment could be impaired if it has been subjected to unfavourable conditions in storage or in transit. -
Page 18: Power Supply Connections
20 Vdc, 5.25 A. The laptop must only be connected to the medical-grade laptop power supply supplied or authorised by Lifelines. Do not use a standard laptop power supply. Only use the laptop supplied or authorised by Lifelines. To avoid the risk of electric shock, this equipment must only be connected to a supply mains with protective earth. -
Page 19: Battery Operation
Do not disassemble, heat above 100°C (212°F) or incinerate. If the Trackit T4A amplifier is not to be used for some time, the battery packs should be removed. The Trackit T4A amplifier is powered from one or two battery packs. When fully charged, two battery packs will typically power the unit for 72 hours depending on the number of channels, sample rate and wireless usage (36 hours if only one battery pack is used). -
Page 20: Use In The Home Environment
The Trackit T4A has an internal Bluetooth radio fitted. This is an approved industry-standard type which present minimal risk of reciprocal interference with other equipment. -
Page 21: Interference
Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Trackit T4A, including cables specified by Lifelines Ltd. Otherwise, degradation of the performance of this equipment could result. -
Page 22: Connections And Usage
Clinical Use During Clinical use, the Trackit T4A Amplifier can be connected to a PC either using the USB cable or through a wireless Bluetooth connection (see Figure 1). Housing the amplifier in the T4A bag is optional and may be used to protect and secure the amplifier. -
Page 23: Laptop Installation And Operation
Home Use During home use, the Trackit T4A Amplifier is battery powered and is housed inside its bag where it is protected against ingress of solid objects and water to a degree of IP22. The laptop PC is optional and may be used for video recordings. -
Page 24: Fitting The Battery Packs
Do not touch the battery contacts (in the Trackit T4A battery compartment) and the patient simultaneously. If the Trackit T4A amplifier is not to be used for some time, the battery packs should be removed. For ambulatory, body-worn applications the Trackit T4A is powered from the battery pack(s). The fully charged battery pack(s) should be fitted into the T4A Amplifier before setting up an ambulatory recording. -
Page 25: Connecting The Trackit T4A Amplifier
Connections and Usage Connecting the Trackit T4A Amplifier The top face of the Trackit T4A houses the display, the patient event pushbutton and the ambient light sensor. For display details refer to page 26. Pressing the pushbutton records a patient event and illuminates the back- light of the display. -
Page 26: Figure 6: Connections And Indicators On The T4A Amplifier, Connector End
1. Patient Event remote switch: this 3.5mm jack connector allows for the connection of an optional Patient Event Thumb Switch. Only the Patient Event Thumb Switch provided by Lifelines should be plugged into this connector. 2. SD memory card slot. -
Page 27: Switching The Amplifier On And Off
Figure 7: Releasing the data cable Bluetooth Connection The Trackit T4A Amplifier has built-in Bluetooth wireless capabilities, which allows the T4A to communicate, wirelessly, with a Bluetooth-enabled PC. This allows the T4A to be monitored remotely over a secure wireless link up to a range of about 100m or greater (dependent on hardware and environmental factors). -
Page 28: Figure 8: Trackit T4A Display
USER MANUAL Connections and Usage Indicators Trackit T4A Display Figure 8: Trackit T4A display The following indicators are shown on the display of the T4A: Symbol Description Clock: Represents the time of day in HH:MM format. When the T4A is connected to the PC, the clock is synchronised to the PC’s clock. -
Page 29: Bluetooth
To pair to a T4A Amplifier: 1. Switch on the T4A amplifier. 2. In the Windows® Bluetooth options, search for new devices. The T4A will be shown as Lifelines T4A – xx, where xx is the serial number of the amplifier. -
Page 30: Figure 9: Bluetooth Pairing
USER MANUAL Connections and Usage 3. Select the desired T4A amplifier and click the “Pair” button. 4. A code will be displayed on the PC and on the T4A, as shown below. Figure 9: Bluetooth pairing 5. If the codes match, press the Event button on the T4A amplifier followed by the ‘Connect’ (Windows 10) or ‘Next’ (Windows 7) button on the PC. -
Page 31: Sd Card
Connections and Usage Bluetooth Usage Once paired, the Trackit T4A acts as the server and provides a serial port service to the PC, acting as a client. The Serial COM port number can be found in the Windows® Bluetooth settings. -
Page 32: Figure 10: Sd Card Location
USER MANUAL Connections and Usage Figure 10: SD Card location Insertion and Removal The T4A Amplifier uses a “push-push” style of SD card holder (push to insert, push to remove). Insertion 1. To insert the card, slide the card into SD card slot (Figure 6, #2) with the SD card label facing up. The card will stop against a spring. -
Page 33: The T4A Bag
USER MANUAL Connections and Usage The T4A Bag The T4A bag provides protection to the T4A amplifier when used in the home environment. The bag features a large zipped opening for fitting the amplifier into the bag. This opening has a fold-over hood to protect the zip from water ingress. -
Page 34: Remote Patient Event Thumb Switch (Optional)
USER MANUAL Connections and Usage Fitting the bag to the patient The bag can be worn by patient over the shoulder or on a belt. Ensure that carrying bag and straps are worn over clothing to prevent any possibility of skin irritation. Strangulation hazard due to long cables. -
Page 35: 3.10 Battery Pack Charging
USER MANUAL Connections and Usage 3.10 Battery Pack Charging The battery pack(s) can be recharged using the supplied single-bay desktop charger (part number 1604) or the optional 4-bay charger (part number 1605). The lithium-polymer battery packs used in this device may present a fire or chemical burn hazard if mistreated. - Page 36 Refer to the instruction sheet included with the charger (which can be found on the Lifelines CD). y Plug the power supply into the socket on the back of the battery charger. Use an appropriate mains lead to connect the power supply to the mains.
- Page 37 USER MANUAL Connections and Usage Each battery compartment has an LED indicator which shows the battery pack’s state of charge, as shown in the table below: LED indicator Description No battery in Charge compartment Slow Flash (once per 1.5 seconds) The battery is charging Battery is too hot or too cold or Fast Flash (5 per second)
-
Page 38: Trackit Software - Overview
Trackit Software - overview The Trackit software is available on the included CD/USB disk or on the Lifelines FTP site. A readme file describes installation. The Trackit Software version 2.8.1.7 (or later) supports the Trackit T4A. Check with your distributor or Lifelines if a newer version of software is available. -
Page 39: The Ambulatory Recording
Eventit.exe program that comes with the Trackit installation. The Trackit T4A can record 15 different event types. These can be seen by looking at the Trackit event types in the online event viewer. See Trackit Events (refer to the Trackit Plus software manual). -
Page 40: Appendix 1: Trackit T4A Amplifier Specifications
USER MANUAL Appendix 1 Appendix 1: Trackit T4A Amplifier Specifications Lifelines reserves the right to change product specifications at any time without notice. This is in-line with the company’s policy of continual product development. EEG inputs Number of EEG channels... - Page 41 USER MANUAL Appendix 1 Bluetooth Wireless Type Bluetooth 4.2 Smart-ready (LE & BR/EDR) Output power 12dBm max. Output frequency 2.402 - 2.480 GHz, ISM band Data rate 1.0 Mbps max. Protocols Standard Bluetooth - SPP, GATT, PAN Modulation GFSK, DQPSK. Frequency Hopping Spread-Spectrum (FHSS) Error correction Forward Error Correction (FEC), Automatic repeat request (ARQ).
- Page 42 MANUAL Appendix 1 Classification of system Classification Clinical use Home use Trackit T4A Amplifier: Internally powered. Internally powered; or it can be Type BF applied parts. Degree of protection against connected to a PC which is powered by electrical shock a medical grade Class I power supply.
-
Page 43: Appendix 2: Additional Events Information
USER MANUAL Appendix 2 Appendix 2: Additional Events Information For the T4A EEG Amplifier, events types are as shown below. y 56 Automatic events (hardware events, photic start/stop, video start/stop etc.) y 40 user-configurable events y Free-text events entered during acquisition Refer to the Trackit Plus software manual for more information Event No Contents... -
Page 44: Appendix 3: Additional Bluetooth Information
USER MANUAL Appendix 3 Appendix 3: Additional Bluetooth Information System overview The Bluetooth module is Bluetooth Qualified v4.2. For full specifications, refer below. Bluetooth is a device-to-computer wireless connection and will connect to any suitably certified Bluetooth host, like a PC or laptop. The connection process uses authentication and password protection. When the Trackit application has established the Bluetooth connection, a connection quality monitor labelled “CommErr”... - Page 45 USER MANUAL Appendix 3 Interference The T4A Amplifier will continue to operate in the presence of radio frequency magnetic fields (RF) and the effects of electrostatic discharges (ESD) and other interference, in accordance with the requirements of IEC60601-1-2. However, the amplifier records signals of very low amplitude, and these signals themselves are not immune to the effects of RF, ESD and low-frequency magnetic field interference.
-
Page 46: Appendix 4: Sd Card Information
BDF File Format The Trackit T4A Amplifier records EEG data to the SD card in BDF format, which is the 24-bit variation of the native EDF (European Data Format). The Trackit software and other EEG applications can view EEG data in BDF format. - Page 47 USER MANUAL Appendix 4 SD Card Capacity Calculation To determine the required SD card capacity, use the following table and calculation. The table below shows the amount of data that will be stored to the card in a 24 hour recording. The number in brackets is the minimum card size that should be used for the selected configuration.
-
Page 48: Appendix 5: Default Setup On Amplifier
USER MANUAL Appendix 5 Appendix 5: Default Setup on Amplifier Physical Signal Range Channel Signal Type Channel Label Units AC Referential Fp1-Ref -375,000 375,000 µV AC Referential Fp2-Ref -375,000 375,000 µV AC Referential F3-Ref -375,000 375,000 µV AC Referential F4-Ref -375,000 375,000 µV... -
Page 49: Appendix 6: Troubleshooting Guide
An incorrect setup has been sent If an incompatible setup has been sent to the Trackit T4A the message; “unable to comply” will indicate that. If an incorrect setup has been sent, the Trackit Control Panel will show ‘ A cquire Ready: No’ . -
Page 50: Appendix 7: Manufacturer's Declaration
Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Trackit T4A, including cables specified by Lifelines Ltd. Otherwise, degradation of the performance of this equipment could result. - Page 51 Guidance and Manufacturer’s Declaration Electromagnetic Emissions IEC 60601-1-2 / EN 60601-1-2 The Trackit T4A is intended for use in the electromagnetic environment specified below. The customer or user of the T4A should assure that it is used in such an environment. Emissions Test...
- Page 52 The Trackit T4A does not contain magnetic components and is not susceptible to power frequency magnetic field interference. f The conditions of intended use justify lower immunity test levels. The hazards and risk analysis associated with these lower limits have...
- Page 53 Appendix 7 Recommended separation distance between portable and mobile RF communications equip-ment and the Trackit T4A EEG System IEC 60601-1-2 / EN 60601-1-2 The T4A is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled.
-
Page 54: Version History
USER MANUAL Version History Version History V 1.0 (2 May 2018) y First release V 1.1 (31 May 2018) y Added power requirements for battery charger y Added warning relating to the battery charger mains isolation y Updated battery compartment image. V 1.2 (26 Nov 2018) y 4-bay battery charger added. - Page 55 Lifelines Ltd, 7 Clarendon Court, Over Wallop, near Stockbridge, Hampshire SO20 8HU, UK Telephone +44 (0)1264 782226 www.LLines.com sales@LLines.com Imagine EEG Anywhere...













Need help?
Do you have a question about the trackit T4A and is the answer not in the manual?
Questions and answers