Vitalograph ALPHA 6000 Instructions For Use Manual
Hide thumbs
Also See for ALPHA 6000:
- User manual (36 pages) ,
- Instructions for use manual (40 pages) ,
- Instructions for use manual (48 pages)
Subscribe to Our Youtube Channel
Summary of Contents for Vitalograph ALPHA 6000
- Page 1 ALPHA MODEL 6000 Instructions for Use © Copyright Vitalograph 2019 Current Edition (Issue 1, 29-Nov-2019) Cat. No. 09080...
- Page 2 Tel: +353 65 6864111 Technical Support Email: technical.support@vitalograph.ie Tel: +353 65 6864111 Email: technical.support@vitalograph.ie Vitalograph GmbH Rellinger Straße 64a Vitalograph Ltd, Hong Kong/China D-20257 Hamburg P.O. Box 812 Germany Shatin Central Post Office Tel: +49 40 547391-40 Hong Kong Fax: +49 40 547391-40 E-mail: sales@vitalograph.cn...
-
Page 3: Table Of Contents
ALPHA - Instructions for Use 09080 Issue 1 Contents Main Components of the Vitalograph ALPHA ........4 1.1. Features of the Vitalograph ALPHA .........4 Setting Up the Vitalograph ALPHA ...........5 2.1. Fitting a New Paper Roll ............6 Operating Instructions ...............7 3.1. Entering Subject Data ..............7 3.2. -
Page 4: Main Components Of The Vitalograph Alpha
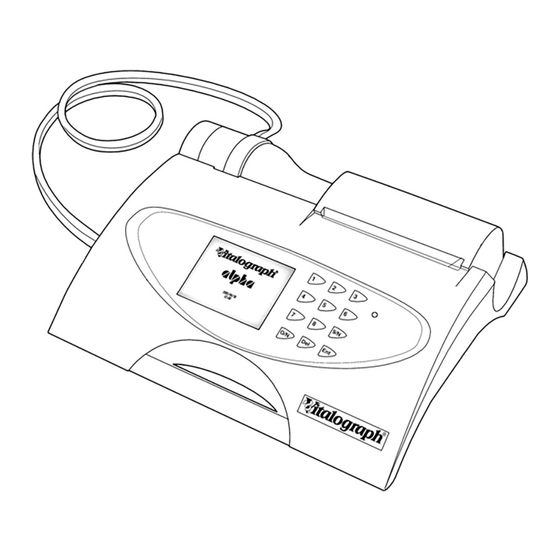
A Global Leader in Respiratory Solutions 1. Main Components of the Vitalograph ALPHA Figure 1 Main components of ALPHA Note: Computer not supplied Display Internal Thermal Printer Flowhead Flowhead Connection Tubing Medically Approved Power Supply BVF™ USB Cable Vitalograph Reports Software (Optional) -
Page 5: Features Of The Vitalograph Alpha
5. The ALPHA can be connected through the USB port at the side of the unit to the user’s computer, so that the report can be written to the Vitalograph Reports Utility (refer to the Vitalograph Reports Instructions for Use) 6. -
Page 6: Fitting A New Paper Roll
Note: To make fitting easier, create a point in the middle of the paper by tearing or cutting the two corners of the leading edge of the paper. Note: The Vitalograph logo should be facing you on the right edge of the paper. -
Page 7: Operating Instructions
ALPHA - Instructions for Use 09080 Issue 1 3. Operating Instructions Turn on the ALPHA (the On/Off switch is at the back of the device). 3.1. Entering Subject Data After a short delay, the Welcome screen displays briefly. This is followed by a screen for entering a new subject’s detail showing the first field to be entered. -
Page 8: Conducting A Test
A Global Leader in Respiratory Solutions To enter another subject’s details, select the ‘New Subject’ option from the Main Menu using the keypad. A dialog box will appear to confirm whether to clear current subject details. • Press ‘Yes’ to open New Subject screen and enter the new subject details. •... - Page 9 ALPHA - Instructions for Use 09080 Issue 1 d. Repeat for a minimum of three manoeuvres, usually no more than eight for adults. e. Check VC repeatability and perform more manoeuvres as necessary. Note: A single-breath VC technique may also be performed on the device. FVC test session: a.
-
Page 10: Reporting
Note: The internal (thermal) printout will fade over time when exposed to light or heat. If a permanent record is required, photocopy the thermal printout or send the report to the Vitalograph Reports Utility. Refer to the section on Configuration for information on selecting the internal or Vitalograph Reports Option. -
Page 11: Calibration Verification
ALPHA - Instructions for Use 09080 Issue 1 Subjects and their associated stored test is presented. A maximum of nine Subject/Tests can be stored on the device in locations 1-9 as indicated in the Delete Subject screen. 3. Use the keypad to select the number of the Subject/Test to be deleted. This location will then be marked as ‘Empty’. -
Page 12: Configuration Options
Set user requirements on the following options: Printer – Select whether to print the test report to the Internal Printer or send to the Vitalograph Reports Utility on a PC. Press key ‘1’ on the keypad to switch between the two options. - Page 13 ALPHA - Instructions for Use 09080 Issue 1 • Press key 1 to switch the Volume/time (V/t) graph on or off. • Press key 2 to switch the Flow/Volume (F/V) graph on or off. • Press key 3 to switch the comments on or off. •...
-
Page 14: Power Management
3.6.5. Calibration Calibration is not required for normal use. Calibration should only be conducted by, or under the guidance of Vitalograph Service Agents. See contact details on p. 2. Select Calibration using the keypad, then calibrate by the following steps: 1. -
Page 15: Battery Low Detect
ALPHA - Instructions for Use 09080 Issue 1 4.2. Battery Low Detect The battery power detect messages are: Battery Low: The icon flashes on and off on the Main Menu screen. The battery pack is running low. You may continue to use the device. -
Page 16: Cleaning & Hygiene
5.1. Preventing Cross-Contamination of Subjects A spirometer is not designed or supplied as a ‘sterile’ device. Vitalograph intends that a new Bacterial Viral Filter (BVF) be used for every subject to prevent cross contamination. Using a new BVF provides a significant level of protection of for the subject, the device and the user against cross contamination during spirometry manoeuvres. -
Page 17: Fault Finding Guide
ALPHA - Instructions for Use 09080 Issue 1 6. Fault Finding Guide Problem Fault • Accuracy check variations > +/-3% Symptoms: • False readings suspected • Recheck Calibration with reference to section Checking Accuracy • Was the correct syringe volume selected? •... -
Page 18: Software Check
• Ensure the green flap on the printer is pressed down. • Internal printer failure – contact support. Problem Fault • Cannot print to PC (Vitalograph Reports Utility). Symptoms: • Corrupt or missing data on printout. • Check that external printer is selected in Configuration screen. -
Page 19: Customer Service
Any serious incident that has occurred in relation to the device should be reported to Vitalograph or its Authorized Representative and the Regulatory Authorities of the country. Refer to the Vitalograph contact information at the start of this manual. 8. Consumables and Accessories Cat. -
Page 20: Explanation Of Symbols
A Global Leader in Respiratory Solutions 10. Explanation of Symbols Symbol Description Type BF equipment Class II Power rating Direct current Instructions for Use; operating instructions Manufacturer Year of Manufacture (Date format YYYY-MM-DD) USB connector The device must be taken to separate collection at the product end-of-life. -
Page 21: Description Of The Vitalograph Alpha
11.1. Indications for Use The indications for use of the Vitalograph ALPHA is in the simple assessment of respiratory function through the measurement of dynamic lung volumes. The Vitalograph ALPHA is designed to be operated by medical professionals trained in respiratory and lung function testing on adults and paediatrics, 2.5... -
Page 22: Technical Specification
A Global Leader in Respiratory Solutions 12. Technical Specification Product Vitalograph ALPHA, model 6000 Flow Detection Principal Fleisch type pneumotachograph Essential Performance Flow measurement output Essential Performance Test Flow Accuracy ±10% or ±20 L/min, whichever Limits is greater Less than 0.1kPa/L/second @ 14L/s,... - Page 23 Operating Systems: Windows® 7, Windows® 8 or Windows® 10 Memory: 128 MB of RAM, 256 MB recommended Hard Disk: 40MB for the Vitalograph Reports application 280MB for the .NET framework Display: A display supporting a resolution of Minimum PC System 1280 x 800 pixels, higher recommended.
-
Page 24: Contraindications, Warnings, Precautions And Adverse Reactions
1. No modification of this equipment is allowed. Any unauthorised changes to the Vitalograph ALPHA device may compromise product safety and/or data and as such Vitalograph cannot be held responsible and the device will no longer be supported. 2. The Vitalograph ALPHA is not designed as a sterile device. Always follow the safety guidelines given by the manufacturer of cleaning and disinfectant chemicals. - Page 25 Vitalograph support. In case of leaking ensure the electrolyte does not get in the eyes or touch skin. If electrolyte contacts the eyes flush the area immediately with water for 15 minutes and seek medical attention.
-
Page 26: Ce Notice
Vitalograph Model 6000 ALPHA to the Medical Devices Directive of the European Community. The Vitalograph Model 6000 ALPHA is intended for use in a variety of professional healthcare environments, e.g. primary care, hospital wards and occupational health centres, except for near active high frequency surgical equipment and the RF shielded room of an ME system for magnetic resonance imaging, where the intensity of electromagnetic disturbance is high. - Page 27 ALPHA - Instructions for Use 09080 Issue 1 EN 60601-1-2:2007 - Immunity tests Immunity test Test level Compliance level Reached Electrostatic ±6 kV contact ±6 kV contact discharge (ESD) ±8 kV air ±8 kV air IEC 61000-4-2 Electrical fast ±2 kV for power supply lines ±2 kV for power supply transient/burst ±1 kV for input/ output lines...
-
Page 28: Fda Notice
It is recommended that all equipment used near the Vitalograph product comply with the medical electromagnetic compatibility standard and to check before use that no interference is evident or possible. If interference is suspected or possible, switching off the offending device is the normal solution, as is required in aircraft and medical facilities. -
Page 29: Eu Declaration Of Conformity
09080 Issue 1 16. EU Declaration of Conformity Product: Model 6000 Vitalograph ALPHA Vitalograph hereby ensures and declares that the above product associated with these instructions for use, is designed and manufactured in accordance with the following QMS regulations and standards: •... -
Page 30: Guarantee
A Global Leader in Respiratory Solutions 17. Guarantee Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its opinion replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials. - Page 31 ALPHA - Instructions for Use 09080 Issue 1 Page of 32...










Need help?
Do you have a question about the ALPHA 6000 and is the answer not in the manual?
Questions and answers