Subscribe to Our Youtube Channel
Summary of Contents for Vitalograph In2itive 2120
- Page 1 Model 2120 Hand Held Spirometer Vitalograph Vitalograph In2itive™ Vitalograph Vitalograph User Manual Page 1 of 45 DT_0006-6...
- Page 2 Tel: (065) 6864100 Fax: (913) 888 4259 Fax: (065) 6829289 e-mail: vitcs@vitalograph.com e-mail: sales@vitalograph.ie Internet: www.vitalograph.co.uk © Copyright Vitalograph 2009, 2010 & 2011 Current Edition (Issue 5) Cat. No. 07514 Vitalograph is a registered trademark Page 2 of 45 DT_0006-6...
- Page 3 Table of Contents DESCRIPTION OF THE VITALOGRAPH DEVICE FEATURES OF THE VITALOGRAPH DEVICE GETTING THE VITALOGRAPH DEVICE READY FOR USE OPERATING THE DEVICE WITH SPIROTRAC V CONNECTING THE REMOTE FLOWHEAD POWER MANAGEMENT IN THE VITALOGRAPH DEVICE ATTERY ATTERY ETECT OPERATING THE VITALOGRAPH DEVICE...
- Page 4 FAULT FINDING GUIDE CUSTOMER SERVICE CONSUMABLES AND ACCESSORIES EXPLANATION OF SYMBOLS TECHNICAL SPECIFICATIONS CE NOTICE FDA NOTICE DECLARATION OF CONFORMITY GUARANTEE Page 4 of 45 DT_0006-6...
- Page 5 LCD and printed and downloaded to a PC. There are a variety of backup and other configuration options. Information about the software can be obtained from the About box. This information can be used if any queries are made to Vitalograph or a service agent. To access the About box: 1.
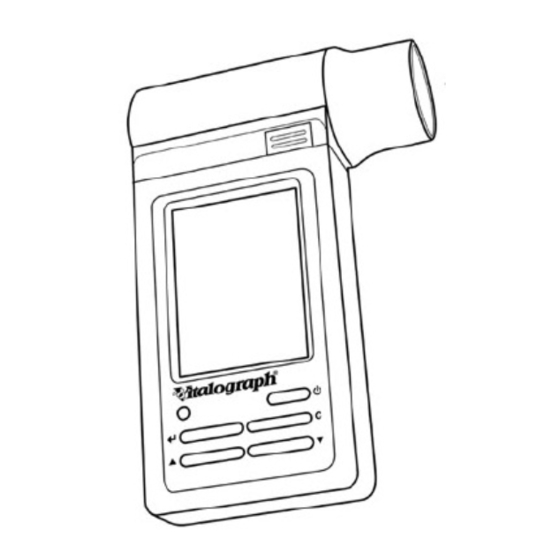
- Page 6 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 PC Software Flowhead Flowhead Release Button LCD/Touch panel Display On/Off Button Mini USB ports in Stylus cradle and device Holder Cancel Button Down Button Up Button Enter Button Figure 1 Page 6 of 45...
- Page 7 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 EATURES OF THE ITALOGRAPH EVICE The Vitalograph In2itive features include: • Fleisch pneumotachograph • Integral and removable flowhead • Touch screen color display or buttons • Clear sounds for audio feedback •...
- Page 8 ONNECTING THE EMOTE LOWHEAD The flowhead on the Vitalograph In2itive can be set up to work remotely from the device. This can be done as follows (see figure 2): 1. Hold the device body firmly in your left hand. 2. Hold the flowhead with your right hand, at the same time press and hold the button firmly on the front of the fleisch flowhead.
- Page 9 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 the top cover. The Vitalograph font and button on the device cap should be on the same face as the LCD when assembled. Ensure this is fully pushed in. 5. Attach the remote flowhead adaptor to the flowhead. This is done by sliding the flowhead into the grooves in the remote flowhead adaptor.
- Page 10 ANAGEMENT IN THE ITALOGRAPH EVICE The Vitalograph In2itive can be powered using the purpose-built low voltage Power Supply unit with which it is supplied or from the PC via the USB cable or from the internal Battery Pack. When powered from the low voltage Power Supply or the PC the LED on the front face on the device will be orange.
-
Page 11: I Nformation
Issue 5 Battery Pack The Vitalograph In2itive is fitted with a rechargeable Battery Pack. This allows the device to be used without the 5V Power Supply connected. The battery pack can be re-charged by plugging in the 5 V Power Supply. -
Page 12: Est S Ession
Before starting a test session, there are a number of checks which should be made: 1. Ensure that the accuracy of the Vitalograph In2itive unit was checked recently. (Refer to the section on Checking Accuracy) 2. Ensure a subject is selected (subject ID or name will appear on the status bar at the bottom of the Main Menu) and the required demographic information is entered for the subject. - Page 13 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 This indicates that the Vitalograph In2itive unit is ready to accept a blow. Note: You can view the results as either a Volume/time graph or a Volume bar graph by selecting the relevant tab.
- Page 14 1. Select the ‘FVC Test’ option from the Main Menu. 2. Wait for the ‘Exhale to Begin’ icon to appear. This indicates that the Vitalograph In2itive unit is ready to accept a blow. Note: You can view the results as either a Volume/time (V/t) or a Flow/Volume (F/V) graph by selecting the relevant tab.
- Page 15 Keep eye contact with the subject) If inspiratory indices are selected, then inhale as quickly as possible. g. Listen for two beeps. This indicates that Vitalograph In2itive is ready for the next blow. Method 2: a. Sit upright, fit the noseclip and relax.
- Page 16 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 The best 3 tests performed and the Test Grade are shown in the V/t screen. Each test series is graded in relation to its repeatability between acceptable manoeuvres. The quality Grades are A, B, C, D and F.
-
Page 17: S Ession
Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 Saving the Test Session. The test session is saved to the database by following the on-screen messages. These messages appear when a new subject, an accuracy check or POST mode is selected. - Page 18 The Vitalograph In2itive can be connected through the USB port at the side of the unit or from the cradle to the Vitalograph Reports Utility, so that the report can be written to a PC. By using the optional Print Cradle the Vitalograph In2itive can also be connected to an external PCL compatible printer.
- Page 19 Issue 5 The information printed on the session report and also the reports method (Vitalograph Reports or External Printer) can be configured to suit individual requirements. Refer to section on Report Options. You can also select the Report option from the Main Menu. If you...
- Page 20 3-L syringe to validate that the instrument is measuring accurately. The Vitalograph In2itive should never be outside accuracy limits unless damaged or in a fault condition. In this event, see the fault-finding guide.
- Page 21 If the flowhead or device has been dropped. Configuration Options There are a number of Configuration options available on the Vitalograph In2itive device. To access these, select the ‘Configuration’ option on the Main Menu. The options available are: Test Preferences This allows you to configure the test screen to your requirements.
- Page 22 The Deletion tab allows the user to delete sessions. By selecting the relevant box the session will be deleted from the device after being Printed. (Either a print to an external printer or sent to Vitalograph Reports). You are also given the option to ‘Select Test Session(s)’ for deletion, or ‘Delete Subject(s)’...
- Page 23 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 b) Pump air through the flowhead to bring it to ambient temperature. If the flowhead has very recently been used for testing or has come from a cold environment, its temperature should be equilibrated with ambient by pumping air through it from the syringe several times.
- Page 24 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 Sound Options This allows you to turn off and on the Key, Flow, Error and Welcome sounds on the device. Simply select the on/off key for the specific sound. Incentive The Incentive Device is used as an aid in testing of children.
- Page 25 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 The following list supplies definitions of the parameters: Parameter Definition Vital capacity (L) Inspiratory vital capacity (L) FIVC Forced inspiratory vital capacity (L) Forced vital capacity (L) FEV.5 Forced expiratory volume after 0.5 seconds (L) FEV.75...
- Page 26 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 Inspiratory capacity (L) Rind Airways Resistance Indirect measurement. FIVC/FVC Ratio FIVC of FVC FEV.5/FVC Ratio FEV 0.5 of FVC FEV1/FEV6 Ratio FEV1 of FEV6 FEV1/FVC Ratio FEV1 of FVC FEV1/VC Ratio FEV1 of VC...
- Page 27 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 on the device. To change the setting simply select the on/off button on the touch panel keypad. c) The device can be password protected, by setting the on/off button to the on position. The password can be changed using the touch panel keypad.
- Page 28 Report Method The In2itive can print to a compatible USB PCL printer or Vitalograph Reports. The following options are available: a) Report: Select the option to either Send To PC or External Printer from the drop down list.
- Page 29 Colour: The printout can be set to Colour or Black & White. Select on for a colour printout, and off for black & white. Note: In order to send the report to the Vitalograph Reports Utility it is necessary to have the Vitalograph Reports Utility installed on your PC and the In2itive connected to your PC via a USB cable.
-
Page 30: V Italograph I N 2 Itive
Issue 5 LEANING NSTRUCTIONS Cleaning and Disinfecting the Vitalograph In2itive A new mouthpiece (either SafeTway or BVF) should be used for each subject. A delay of at least 5 minutes should be allowed between subjects to allow settling of previously aerosolized particles in the measuring device. - Page 31 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 Table of Materials Used & Cleaning/Disinfection Methods This listing of materials used is given to provide users with information to allow the assessment of other cleaning and disinfecting procedures available in the facility on this device.
-
Page 32: Leisch F Lowhead
Note: Always follow the safety guidelines given by the manufacturer of cleaning and disinfectant chemicals. All external parts of the Vitalograph In2itive require cleaning, i.e. the removal of visible particulate contamination. The parts of the Vitalograph In2itive that make up the flowhead, which comes into contact with subjects being tested, also require disinfecting. -
Page 33: F Leisch F Lowhead
3. Fit a new flowhead cone to the flowhead. 4. Slide the flowhead into the grooves in the top cover. The Vitalograph logo and button on the flowhead should be on the same face as the LCD when assembled. 5. It is recommended that an accuracy check is carried out following reassembly to verify correct operation and accuracy. - Page 34 • Check printer compatibility – contact support. • Main PCB failure – contact support. • Cannot print to PC (Vitalograph Reports Problem Fault Symptoms: Application). • Corrupt or missing data on printout. • Check that Send to PC is selected in the...
- Page 35 Service and repairs should be carried out only by the manufacturer, the approved importer or by Service Agents specifically approved by Vitalograph. For the names and addresses of approved Vitalograph Service Agents or to arrange spirometry workshops, please refer to the contact information at the start of this manual.
- Page 36 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 ONSUMABLES AND CCESSORIES Cat. no Description 20242 SafeTway Mouthpieces (200) 20303 Nose Clips (200) 28350 BVF - Bacterial/Viral Filters (50) 36020 3-L Precision Syringe 79158 Flow Cone (10) 40079 Mini USB Cable...
- Page 37 Communications USB, micro SD Card, Cradle Notes: - All values displayed by the Vitalograph In2itive are expressed as BTPS values. - Take care not to block the mouthpiece with the tongue or teeth. A ‘spitting’ action or coughing will give false readings.
- Page 38 Marking by the symbol indicates compliance of the Vitalograph In2itive to the Medical Devices Directive of the European Community. Such marking is indicative that the Vitalograph In2itive meets or exceeds the following technical standards: Guidance and manufacturer’s declaration – electromagnetic emissions The Model 2120 is intended for use in the electromagnetic environment specified below.
- Page 39 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 Guidance and manufacturer’s declaration – electromagnetic immunity The Model 2120 is intended for use in the electromagnetic environment specified below. The customer or the user of the Model 2120 should assure that it is used in such an environment...
- Page 40 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 for 25 cycles <5 % 100V (>95 100V) for 5 sec 3 Α/m Power frequency Applicable (50/60 magnetic field IEC 61000-4-8 Guidance and manufacturer’s declaration – electromagnetic immunity The Model 2120 is intended for use in the electromagnetic environment specified below.
- Page 41 Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 80 MHz to 80MHz 61000-4-3 2.5 GHz 2.5GHz d = 2.3√P…800 MHz to 2.5GHz Where P is the maximum output power rating of the transmitter in Watts (W) according to the transmitter...
- Page 42 Medical Devices may be affected by cellular telephones and other personal or household devices not intended for medical facilities. It is recommended that all equipment used near the Vitalograph product comply with the medical electromagnetic compatibility standard and to check before use that no interference is evident or possible. If...
-
Page 43: Fda Notice
Vitalograph In2itive User Manual Cat. No. 07514 Issue 5 interference is suspected or possible, switching off the offending device is the normal solution, as is required in aircraft and medical facilities. Medical electrical equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC... - Page 44 ECLARATION OF ONFORMITY Model 2120 Hand Held Spirometer Product Vitalograph In2itive™ Vitalograph hereby ensures and declares that the above product associated with this user manual, is designed and manufactured in accordance with the following QMS regulations and standards: •...
- Page 45 5. If a defect occurs please contact the supplier from it was purchased for advice. The Company does not authorize any person to create ® for it any other obligation or liability in connection with Vitalograph equipment. 6. This Guarantee is not transferable and no person, firm or company has any authority to vary the terms or conditions of this guarantee.








Need help?
Do you have a question about the In2itive 2120 and is the answer not in the manual?
Questions and answers