Subscribe to Our Youtube Channel
Summary of Contents for Vitalograph Micro 6300
- Page 1 MODEL 6300 Instructions for Use © Copyright Vitalograph 2022 Current Edition (Issue 2, 29-Jun-2022) Cat. No. 09360...
- Page 2 Tel: +49 40 547391-0 Fax: +49 40 547391-40 E-mail: info@vitalograph.de www.vitalograph.de Technical Support Telefon: +49 40 547391-14 E-mail: support@vitalograph.de © Copyright Vitalograph 2022 Current Edition (Issue 2, 29-Jun-2022) Cat. No. 09360 Vitalograph is a registered trademark. Page of 44 DT_0006 Issue 16...
-
Page 3: Table Of Contents
1. Indications for Use ..............4 2. Contraindications, Warnings, Precautions and Adverse Reactions ................. 4 3. Main Components of the Vitalograph micro ......7 3.1. Features of the Vitalograph micro ......8 4. Setting Up the Vitalograph micro ........... 8 5. -
Page 4: Indications For Use
2. The micro is not designed as a sterile device. Always follow the safety guidelines given by the manufacturer of cleaning and disinfectant chemicals. 3. Vitalograph intends that a new Bacterial Viral Filter (BVF™) be used for every subject to prevent cross contamination. Using a new BVF provides a significant level of protection of the subject, the device, and the user against cross contamination during spirometry manoeuvres. - Page 5 15. Service and repairs should be carried out only by the manufacturer or by Service Agents approved by Vitalograph. 16. Maintenance must not be performed while the device is in use by a subject.
- Page 6 During normal use, these edges are covered, are not accessible by the user or subject. 23. Use of accessories and cables other than those specified or provided by Vitalograph for this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of the device and result in improper operation. 24. Non-medical equipment must be kept outside the subject environment i.e., any area in which intentional or unintentional...
-
Page 7: Main Components Of The Vitalograph Micro
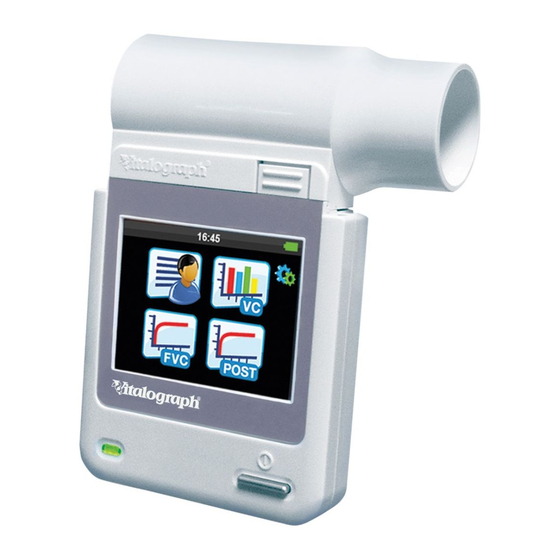
There are no adverse effects if the subject comes into contact with any other part of the device. 3. Main Components of the Vitalograph micro The micro is a standalone spirometer. Device Studio software allows the micro to generate reports to a computer after testing is complete, but it is not required for the device to function. The main... -
Page 8: Features Of The Vitalograph Micro
A Global Leader in Respiratory Solutions 3.1. Features of the Vitalograph micro The features include: • Fleisch Pneumotachograph • Removable flowhead • Touch screen colour display • Choice of predicted values • Report generation through Device Studio software • Storage of tests and demographic information 4. -
Page 9: Operating Instructions
Vitalograph micro - Instructions for Use 09360 Issue 2 gripping and sliding it firmly in the direction of the flowhead cone (6). 2. Connect the remote flowhead adaptor (4) to the base of the flowhead (5), connect the device cap (2) to the device (1). 3. Unwrap the flowhead connection tubing (3) and connect one end to device cap (2) and the other end to the remote flowhead adaptor (4). See Figure 2. The tubing is keyed, so it will only connect one way. Figure 2 micro with remote flowhead... -
Page 10: Entering Subject Data
A Global Leader in Respiratory Solutions Press the forward button to save. Continue to the Main Menu screen which includes the following options: New Subject VC Test FVC Test Post Test The test icons will appear greyed out and can’t be selected until a subject is created. The Post Test icon becomes active when an FVC pre-test is performed. -
Page 11: Conducting A Test
Vitalograph micro - Instructions for Use 09360 Issue 2 6. Press the forward button to save the subjects detail 7. If a value is not entered for Birth Sex, Height or Date of Birth then an Error Icon will appear next to the empty field. If the information is not entered then the predicted values will not appear in test results. - Page 12 A Global Leader in Respiratory Solutions 3. Then exhale in a relaxed manner with no hesitation until no more air can be expelled. It is vital that the operator encourages the subject to keep exhaling to ensure all air is expelled (when a plateau has been reached or expiration time reaches 15 seconds).
- Page 13 Vitalograph micro - Instructions for Use 09360 Issue 2 Note: Single breath technique may also be performed. 5.2.2. FVC Test To Perform an FVC test 1. Instruct the subject to breathe normally. 2. The subject should inhale completely and rapidly, with a brief pause when lungs are completely full (≤ 2 secs).
- Page 14 A Global Leader in Respiratory Solutions explains the order of the tests. 13. Select on the side menu to view results. • Use the left/right arrow to select which test to view. • Scroll through the results for each test using the up/ down arrows.
- Page 15 Vitalograph micro - Instructions for Use 09360 Issue 2 between tests so that they are registered as separate sessions and separate reports can be generated. Note: A session ends and is saved when one of the following occur: the device is turned off, a new subject is created, the device is connected to Device Studio.
-
Page 16: Reporting
A Global Leader in Respiratory Solutions Note: If more than the maximum subject/session entries are stored on the device the existing subject/sessions entries will be deleted on a First In First Out (FIFO) basis i.e. the first session entered will be the first to be deleted. -
Page 17: Calibration Verification
5.4. Calibration Verification The Vitalograph micro should never be outside accuracy limits unless damaged or in a fault condition. In normal use, it is recommended that a daily calibration verification is performed on the device. ISO 26782 recommendations require that the difference between the volume measured by the spirometer and the volume pumped into the spirometer from a syringe is within 3%. - Page 18 A Global Leader in Respiratory Solutions 6. The result of each stroke, expiratory (E) and inspiratory (I) displays on the top of the screen with the number of strokes shown in between. If they are reproducible and within 3% this will be displayed on the top of the screen, and a syringe with a green tick pass will display.
-
Page 19: Configuration Options
5.3 Reporting. If the procedure was followed correctly and the error icon is showing, the calibration verification should be repeated. If the error continues to show, contact Vitalograph using the contact information at the start of this document. Note: To exit the Calibration Verification screen without performing a check, press the forward key again to return to the Configuration... - Page 20 A Global Leader in Respiratory Solutions a. Posture: set the posture for the session to sitting standing b. Weight: turn on to enter the subject’s weight or off if not required. c. Population Group: turn on to enter the subject’s population group or off if not required. The Device settings option to configure: ...
- Page 21 Vitalograph micro - Instructions for Use 09360 Issue 2 Forced vital capacity (L) FEV1 Forced expiratory volume after 1 second (L) FEV1 divided by the largest VC from the VC or FVC FEV1R manoeuvre. PEF L/s Peak expiratory flow (L/sec) PEF L/min Peak expiratory flow (L/min)
- Page 22 A Global Leader in Respiratory Solutions FEV3/VC Ratio FEV3 of FVC FEV6 Forced expiratory volume after 6 seconds (L) FEF25 Forced expiratory flow at 25% of the FVC (L/sec) FEF50 Forced expiratory flow at 50% of the FVC (L/sec) FEF75 Forced expiratory flow at 75% of the FVC (L/sec) Mean forced expiratory flow in the volume interval FEF0.2-1.2 between 0.2 and 1.2 L of the test (L/sec) FEF 25- Ratio FEF25-75 of FVC 75/FVC FIV1/FVC Ratio FIV1 of FIVC...
-
Page 23: Power Management
Vitalograph micro - Instructions for Use 09360 Issue 2 c. Date/Time: Select to set or change the date and/or time. Use the up/down arrows to edit fields. d. Service mode/Technician. This option is for servicing and technicians, a passcode is required to activate. Calibration Verification : See section 5.4 for details on performing a Calibration Verification. About : Contains information about the software which should be used if making enquiries to Vitalograph or a service agent. -
Page 24: Power Save Mode
7. Cleaning & Hygiene 7.1. Preventing Cross-Contamination of Subjects A spirometer is not designed or supplied as a ‘sterile’ device. Vitalograph intends that a new Bacterial Viral Filter (BVF) be used for every subject to prevent cross contamination. Using a new BVF provides a significant level of protection for the subject, the device and the user against cross contamination during spirometry manoeuvres. - Page 25 Vitalograph micro - Instructions for Use 09360 Issue 2 Where the user suspects that the flowhead has become contaminated or where local risk assessment identifies a need for higher level of decontamination, then it should be cleaned as per the instructions in ‘Cleaning and Hygiene’ on the Vitalograph website. Table of Cleaning/Disinfection Methods Clean/ Recommended Cleaning/Low Part Low Level...
-
Page 26: Inspection Of The Vitalograph Micro
Examine the Fleisch element and replace if damaged. If it is suspected that the flowhead has become contaminated or where user risk assessment identifies a need for higher level of decontamination, then it should be cleaned as per the instructions in ‘Cleaning and Hygiene’ on the Vitalograph website. It is recommended that a calibration verification is carried out following cleaning and re-assembly as recommended by the ATS/ ERS 2019 guidelines... -
Page 27: Fault Finding Guide
Vitalograph micro - Instructions for Use 09360 Issue 2 8. Fault Finding Guide • Calibration verification variations > Problem Fault +/-3% Symptoms: • False readings suspected • The % error indicates the severity of the issue. Over 3%, repeat the calibration verification. Over 25%, the user should... -
Page 28: Software Check
• Electronics failure. Contact support. 8.1. Software Check Information about the device may be obtained from the About option in the Configuration menu. This information may be used when enquiries are made to Vitalograph or a service agent. 8.2. Product Useful Life Checks To ascertain whether the device has exceeded its useful life Vitalograph recommends checking the flowhead and the real time clock. Page... -
Page 29: Customer Service
Any serious incident that has occurred in relation to the device should be reported to Vitalograph or its Authorised Representative and the Regulatory Authorities of the country. Refer to the Vitalograph contact information at the start of this document. -
Page 30: Disposal
A Global Leader in Respiratory Solutions 41660 LCD Spare 41659 Main PCBA Spare 83157 Flowhead Spare 63660 Service Kit 83200 Flowhead adaptor kit 11. Disposal The device is marked with the WEEE symbol and must be taken to separate collection at the product end-of-life. Do not dispose of these products or the batteries as unsorted municipal waste, they should be disposed of in line with local requirements. -
Page 31: Description Of The Vitalograph Micro
MR Unsafe – Do not use this device in an MRI environment. 13. Description of the Vitalograph micro The Vitalograph micro is a handheld spirometer which measures subject respiratory parameters. It is designed for portable spirometry but may be connected to the associated Device Studio application to view and print reports. The Fleisch flowhead is used... -
Page 32: Technical Specification
A Global Leader in Respiratory Solutions 14. Technical Specification Product Vitalograph micro, Model 6300 Flow Detection Principal Fleisch type pneumotachograph Flow integration sampling @ Volume detection 100Hz Maximum test duration 90 seconds Maximum displayed 10 L volume Volume Accuracy ±2.5% Max. flow rate ±960 L/min (±16... - Page 33 Vitalograph micro - Instructions for Use 09360 Issue 2 142mm (length) x 81mm (width) Dimensions x 24mm (height) 260g (device only, no batteries, Weight packaging or accessories) USB 2.0 Communications Bluetooth 2/4 5V DC via USB Power Supply 4 x 1.5V AAA batteries (6V)
-
Page 34: Ce Notice
15. CE Notice Marking by the symbol indicates compliance of the Vitalograph Model 6300 micro to the Medical Devices Directive of the European Community. The micro is intended for use in a variety of healthcare environments, e.g. - Page 35 Vitalograph micro - Instructions for Use 09360 Issue 2 customer or the user of the micro should assure that it is not used in such an environment. The Model 6300 micro has been tested in accordance with: EN60601-1:2006 + A1:2013 - Medical electrical equipment. General...
- Page 36 A Global Leader in Respiratory Solutions The Model 6300 micro is suitable for use in all establishments, including RF emissions domestic establishments Class B CISPR 11 and those connected to the public mains network (e.g. at home and doctor’s offices in residential areas) EN 60601-1-2:2015 + A1:2021 - Immunity tests Compliance level Immunity test Test level...
-
Page 37: Fda Notice
Vitalograph micro - Instructions for Use 09360 Issue 2 Power Frequency Magnetic Field 30A/m 30A/m Immunity EN 61000-4-8 8A/m 30kHz 8A/m 30kHz Power Magnetic Field 65A/m 134.2 k 65A/m 134.2 k Hz Immunity Hz (2.1 kHz PM) (2.1 kHz PM) EN 61000-4-8 7.5A/m 13.56 M... -
Page 38: Eu Declaration Of Conformity
A Global Leader in Respiratory Solutions 17. EU Declaration of Conformity Product: Vitalograph Model 6300 micro Vitalograph hereby ensures and declares that the above product associated with these instructions for use, is designed and manufactured in accordance with the following QMS regulations... -
Page 39: Guarantee
Vitalograph micro - Instructions for Use 09360 Issue 2 18. Guarantee Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its option replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials. - Page 40 A Global Leader in Respiratory Solutions connection with Vitalograph® equipment. 7. This Guarantee is not transferable and no person, firm or company has any authority to vary the terms or conditions of this guarantee. 8. To the maximum extent permitted by law, the Company does not accept liability for any consequential damages arising out of the use of, or inability to use any Vitalograph® equipment. 9. This Guarantee is offered as an additional benefit to the Consumer’s statutory rights and does not affect these rights in any way.
- Page 41 Vitalograph micro - Instructions for Use 09360 Issue 2 Page of 44...
- Page 42 A Global Leader in Respiratory Solutions Page of 44 DT_0006 Issue 16...
- Page 43 Vitalograph micro - Instructions for Use 09360 Issue 2 Page of 44...








Need help?
Do you have a question about the Micro 6300 and is the answer not in the manual?
Questions and answers