Table of Contents
Advertisement
Quick Links
Manufactured for Zewa, Inc.
12960 Commerce Lakes Drive#29
Fort Myers, FL 33913 USA
www.zewa.com
Toll Free Customer Service:
1-888-993-3592
warranty@zewa.com
User Manual
Wrist Blood Pressure Monitor
Thank you very much for selecting ZEWA Wrist Blood Pressure Monitor
WS-380.
To use the monitor correctly and safely, please read the manual thoroughly.
Please keep this manual well in order to reference in future.
Version:1.0
WS-380
Wrist Type
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for zewa WS-380
- Page 1 Wrist Type Manufactured for Zewa, Inc. 12960 Commerce Lakes Drive#29 Fort Myers, FL 33913 USA Thank you very much for selecting ZEWA Wrist Blood Pressure Monitor www.zewa.com WS-380. Toll Free Customer Service: To use the monitor correctly and safely, please read the manual thoroughly.
-
Page 2: Table Of Contents
CATALOGUE INTRODUCTION ..................... General Description Indications for Use Contraindications Measurement Principle Safety Information LCD Display Symbol Monitor Components List BEFORE YOU START ..................Installing and Replacing the Batteries Setting Date, Time and Measurement Unit Select the User ID MEASUREMENT ....................Tie the Cuff Start the Measurement DATA MANAGEMENT... -
Page 3: Introduction
60 records for one user Indications for Use The ZEWA Blood Pressure Monitor is a digital monitor intended for use in measuring blood pressure and heartbeat rate with a wrist circumference ranging from 13.5cm to 21.5 cm ( about 5⅓˝-8½˝ ). It is intended for adult indoor use only. -
Page 4: Safety Information
INTRODUCTION INTRODUCTION Safety Information CAUTION The signs below might be in the user manual, labeling or other component. They are the requirement of standard and using. * This device is intended for adult use in homes only. * The device is not suitable for use on neonatal patients, pregnant women,patients with implanted, Symbol for “THE OPERATION Symbol for “TYPE BF APPLIED electronical devices, patients with pre-eclampsia, premature ventricular beats, atrial fibrillation,... - Page 5 INTRODUCTION INTRODUCTION CAUTION CAUTION * The patient can measure ,transmit data and change batteries under normal circumstances and maintain *On the rare occasion of a fault causing the cuff to remain fully inflated during measurement, open the cuff the device and its accessories according to the user manual. immediately.
-
Page 6: Lcd Display Symbol
* If you have any problems with this device, such as setting up, maintaining or using, please contact the SERVICE PERSONNEL of ZEWA. Don’t open or repair the device by yourself in the event of malfunctions. The device must only be serviced, repaired and opened by individuals at authorized sales/service centers. -
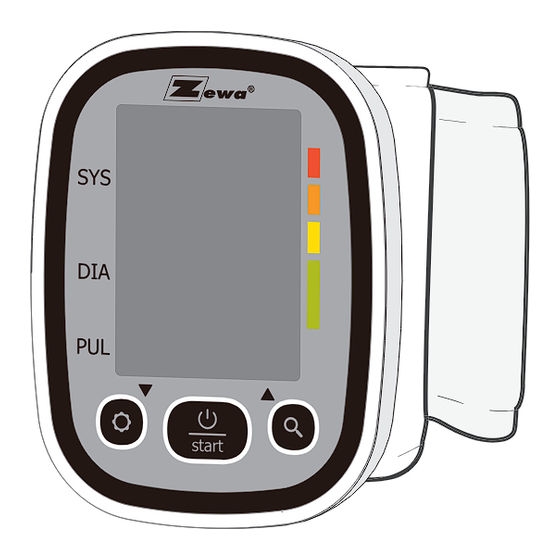
Page 7: Monitor Components
INTRODUCTION INTRODUCTION Monitor Components SYMBOL DESCRIPTION EXPLANATION Measurement unit of the blood pressure Measurement unit of the blood pressure (1kPa=7.5mmHg) Measurement unit the blood pressure mmHg (1mmHg=0.133kPa) Blood pressure monitor is detecting an Irregular heartbeat irregular heartbeat during measurement. SYSTOLIC Month/Day,Hour/Minute Current time GRADE... -
Page 8: List
Install the batteries by matching the correct polarity, as shown 3. Pump; below. Always use the correct battery type (2×AAA batteries.) CUFF 4. Valve; (Type BF applied • Replace the cover. 5. Cuff. part) BATTERY COMPARTMENT List 1) Blood Pressure Monitor WS-380 2) 2×AAA batteries 3) User manual... - Page 9 BEFORE YOU START BEFORE YOU START Setting Date, Time, Positioning Symbol and Measurement Unit Replace the batteries whenever the below happens It is important to set the clock before using your blood shows pressure monitor, so that a time stamp can be assigned to The display is dim.
- Page 10 BEFORE YOU START BEFORE YOU START 4.Repeat step 2 and 3 to confirm [MONTH] and [DAY]. Notes: 1.During the process of setting, you can press “ ” button to stop setting at any time. 2. If there is no operation during the process of setting, it will turn off within 1 minute.
-
Page 11: Select The User Id
BEFORE YOU START BEFORE YOU START Select the User ID .Repeat step 2 and 3 to confirm the measurement unit. Before you start the measurement, please select the desired user ID first. When the blood pressure monitor is off, press and hold “ ”... -
Page 12: Measurement
BEFORE YOU START MEASUREMENT Tie the Cuff Press “ SET ” button to confirm your selection,the LCD will display “User Remove all accessories (watch, bracelet,etc) from your wrist. If your physician has diagnosed you with ID+donE” and then turn off. poor circulation in your wrist, use the other one. -
Page 13: Start The Measurement
MEASUREMENT MEASUREMENT Start the Measurement 2.After finishing the measurement, the LCD displays the blinking icon ‘ ’,and transmits the data. When the monitor is off, press “ ” button to turn on the monitor, and it will finish the whole measurement. (Take user 1 for example.) Note: (1). -
Page 14: Data Management
Interference may occur in the vicinity of equipment marked with the following symbol . And button to show the average value of the WS-380 may interfering vicinity electrical equipment. latest three measurement records. If the Sensitive people, including pregnant women pre-eclamptic and those who implanted medical electronic instruments, should avoid using the unit whenever possible. -
Page 15: Delete The Records
DATA MANAGEMENT DATA MANAGEMENT DATA MANAGEMENT Delete the Records If you want to check the other user’s If you did not get the correct measurement, you can delete measurement records, please press all the results for the selected user by following steps. “... -
Page 16: Information For User
DATA MANAGEMENT INFORMATION FOR USER Tips for Measurement If there is no record, press “ ” button he below display will be shown. Measurements may be inaccurate if taken in the following circumstances. Within 1 hour Immediate measurement Within 20 minutes after dinner or drinking after tea, coffee, smoking after taking a bath... -
Page 17: Maintenance
INFORMATION FOR USER ABOUT BLOOD PRESSURE What are systolic pressure and diastolic pressure? Maintenance When ventricles contract and pump blood out of the heart, blood pressure reaches its maximum To obtain the best performance, please follow the instructions below. value, the highest pressure in the cycle is known as systolic pressure. When the heart relaxes between heartbeats, the lowest blood pressure is diastolic pressure. -
Page 18: Irregular Heartbeat Detector
ABOUT BLOOD PRESSURE ABOUT BLOOD PRESSURE Why does my blood pressure fluctuate throughout the day? Irregular Heartbeat Detector An irregular heartbeat is detected when a heartbeat rhythm varies while the device is measuring 1. Individual blood pressure varies multiple times everyday. It is also systolic pressure and diastolic pressure. -
Page 19: Troubleshooting
TROUBLESHOOTING TROUBLESHOOTING This section includes a list of error messages and frequently asked questions for PROBLEM SYMPTOM CHECK THIS REMEDY problems you may encounter with your blood pressure monitor. If the product is not operating as you think it should, check here before arranging for servicing. A calibration error occurred. -
Page 20: Specifications
SPECIFICATIONS SPECIFICATIONS Power supply Mode of operation Battery powered mode: 2*AAA batteries Continuous operation Display mode Digital LCD V.A.45mmx33mm Degree of protection Type BF applied part Measurement mode Oscillographic testing mode Device Classification Internally Powered ME Equipment Rated cuff pressure: 0mmHg~299mmHg(0kPa ~ 39.9kPa) IP22: The first number 2: Protected against solid foreign Measurement range... -
Page 21: Contact Information
COMPLIED STANDARDS LIST FCC Statement Contact Information For more information about our products or online support, please visit www.zewa.com. To FCC ID: OU9TMB159 contact our customer service, please call 888-993-3592 or support@zewa.com. This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device must accept Manufactured for Zewa, Inc. -
Page 22: Complied Standards List
COMPLIED STANDARDS LIST COMPLIED STANDARDS LIST Complied Standards List EN 1060-3:1997+A2:2009 Non-invasive sphygmomanometers - Part 3: Supplementary requirements for electro-mechanical blood pressure EN ISO 14971:2012 / ISO 14971:2007 Medical devices - measuring systems Risk management Application of risk management to medical devices IEC 80601-2-30:2009+A1:2013 Medical electrical equipment- Part 2-30: Particular requirements for the basic safety and essential EN ISO 15223-1:2016 / ISO 15223-1:2016 Medical devices. -
Page 23: Emc Guidance
30 cm (12 inches) to any part of Harmonic emissions Not application the equipment WS-380, including cables specified by the manufacturer. Otherwise, degradation IEC 61000-3-2 of the performance of this equipment could result. - Page 24 EMC GUIDANCE EMC GUIDANCE Table 2 Power frequency 30 A/m 30 A/m magnetic field 50Hz/60Hz 50Hz/60Hz Guidance and manufacturer’s declaration – electromagnetic Immunity IEC 61000-4-8 Conduced RF Not application Not application Immunity Test IEC 60601-1-2 IEC61000-4-6 Compliance level Test level 10 V/m 10 V/m Radiated RF...
- Page 25 EMC GUIDANCE EMC GUIDANCE Table 3 GSM 1800; Pulse 1720 1700- CDMA 1900; modulation b) 1990 Guidance and manufacturer’s declaration - electromagnetic Immunity GSM 1900; 217Hz 1845 DECT; LTE Band 1, IMMUNITY Radiated RF Test Band Service Modulation Modulation Distance (m) TEST 1970 IEC61000-4-3...







Need help?
Do you have a question about the WS-380 and is the answer not in the manual?
Questions and answers