Table of Contents
Advertisement
Quick Links
Type "B"
Applied Part
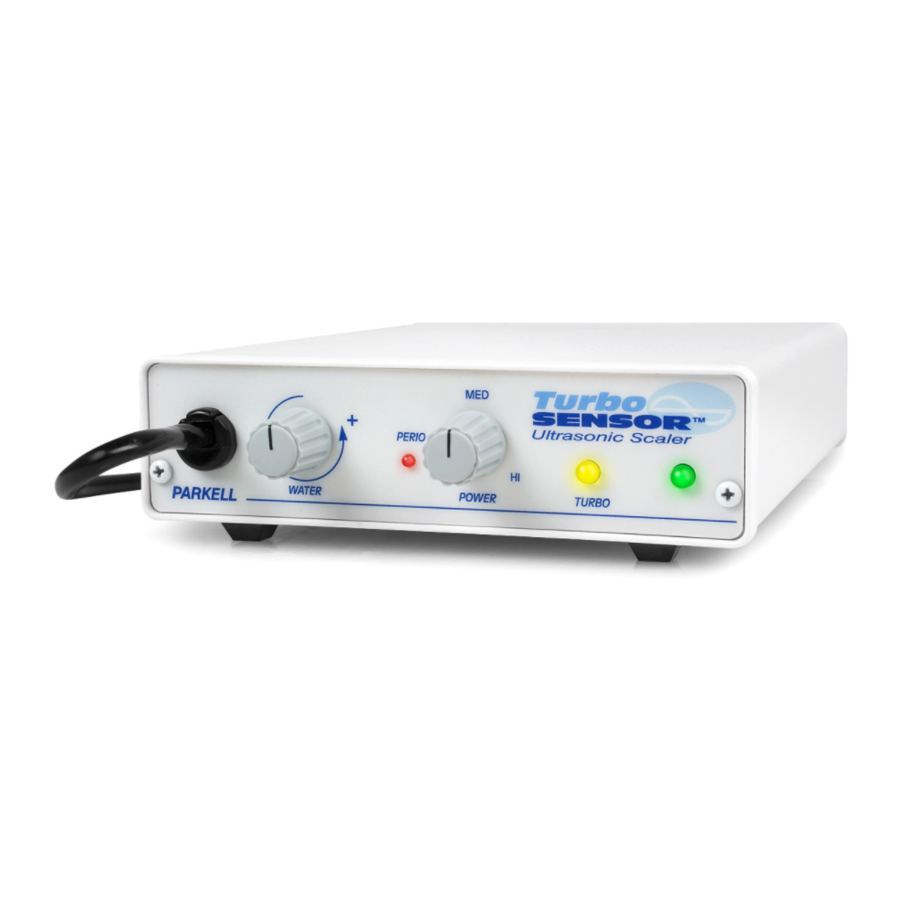
TurboSENSOR
Ultrasonic Tooth Scaler
DEVICE DESCRIPTION:
The TurboSENSOR is an autotune
ultrasonic tooth scaler that operates at either
25KHz or 30KHz. The TurboSENSOR
automatically detects whether the handpiece
contains a 25KHz or 30KHz insert and
switches operating frequency to match.
A dramatically expanded low-power range
improves comfort during debridement.
INTENDED USE/INDICATIONS:
For removal of calculus and plaque during
dental prophylaxis.
WARNING: As with all electrical
products, the unit should not be immersed
in water or other liquids. Do not reach for
the device if it has fallen into liquid. Do
not use the device after it has fallen into
liquid (return it to Parkell for servicing.)
Do not modify this device. Modification
may violate safety codes and endanger
patient and operator. Any modification
will void the warranty.
CONTRAINDICATIONS:
• Do not use this device if the patient or the
operator possesses a cardiac pacemaker
or any other intra-corporeal medical
electronic device (eg. insulin pump,
defibrillator, etc.).
PRECAUTIONS:
Caution: Water flow through tip during use
must be sufficient to cool handpiece and insert.
Caution: Do not allow prolonged contact of
tip with lips, cheek or tongue.
Caution: All ultrasonic scalers produce
aerosols. Take precautions to prevent
transmission of contagious diseases.
Caution: Use the long axis of the insert tip
to wipe accretions from the tooth. Do not
gouge the tooth with the tip.
IMPORTANT: The water supply line
to the scaler should always be turned off
whenever the device is being connected,
disconnected, or when not in use.
Caution: Precautions should be taken to
locate the transformer away from any sources
of water that may enter the unit.
Caution: Federal Law restricts this device to sale by or
on the order of a properly licensed practitioner.
INSERT NOT
INCLUDED
™
INDIVIDUALIZATION OF TREATMENT:
If patient or operator is pregnant, consult
physician concerning advisability of using ultra-
sonic scalers.
Like all scalers of this type, the handpiece
produces a small magnetic field. It has been
reported that this can cause some sensitive
patient monitoring devices (eg. Pulse Oximeter)
to give inconsistent or inaccurate readings.
CONFORMANCE TO STANDARDS:
The Parkell TurboSENSOR conforms to
UL60601-1. Parkell's quality system is
certified to ISO9001/13485. Certified to
CAN/CSA C22.2 No. 601.1.
The device is CE marked - certified to
European Medical Device Directive.
You receive ...
HOW SUPPLIED:
• A Scaler Unit with attached handpiece,
foot controller, water line with male
quick-connect coupler and spare
autoclavable sheath.
• In-line water filter (Stock No. D419)
• Transformer with cable (Stock No.
D411/GS257-120V, D412/GS438-230V)
• Instructions
Does not include inserts. This scaler will
accept any magnetostrictive scaler insert
manufactured by Parkell, Cavitron/Dentsply
Hu-Friedy
®
, or other compatible manufacturers.
SPECIFICATIONS:
I Operating frequency - 25KHz and 30KHz
I Operates at room temperature between 50°F
and 100°F (10°C - 38°C)
I Weight-35 oz.; Size-1
I Power: l20 V, 60Hz, 120VA
(for 230V model: 50Hz, 120VA)
INSTALLING YOUR SCALER:
Locate the device where the control
panel will be easy to reach during scaling
procedures. The scaler requires access to a
grounded electrical outlet and a source of
drinking-quality water.
The scaler and its separate transformer
generate a minimal amount of heat. Avoid cover-
ing them, as this will prevent normal cooling.
®
,
1
1
" H x 5
/
/
"W x 7"D
2
2
Advertisement
Table of Contents

Summary of Contents for parkell TurboSENSOR
- Page 1 You receive ... HOW SUPPLIED: not use the device after it has fallen into liquid (return it to Parkell for servicing.) • A Scaler Unit with attached handpiece, Do not modify this device. Modification foot controller, water line with male...
- Page 2 CONTROLLING THE POWER: The male quick-connect that comes on The TurboSENSOR gives you two ways to the end of the water hose is the same as that adjust the scaling power: a traditional power currently fitted to the Cavitron ®...
- Page 3 If you have any questions, please contact insert may be clogged. Parkell’s Service Dept. at (1-800-243-7446). INFECTION CONTROL: Replacement Filters (10 per package): When removing the autoclavable handpiece Stock No.
- Page 4 Insert does not vibrate properly: I Faulty, damaged or worn insert. to Parkell. The unit will be repaired and I Insert not correctly seated in handle. returned to the purchaser. I Power control not correctly adjusted.






Need help?
Do you have a question about the TurboSENSOR and is the answer not in the manual?
Questions and answers