Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Arjohuntleigh FLOWTRON HYDROVEN 3
- Page 1 FLOWTRON HYDROVEN 3 Instructions for Use 0086 ...with people in mind...
-
Page 3: Table Of Contents
Flowtron Hydroven 3 System ........ - Page 4 (ii)
-
Page 5: General Safety
• Keep the pump away from sources of liquids and do not immerse in water. • Do not use the pump in the presence of uncontained flammable liquids or gasses. • Only the pump and garment/insert combination as indicated by ArjoHuntleigh should be used. The correct function of the product cannot be guaranteed if incorrect pump and garment combinations are used. - Page 6 ArjoHuntleigh distributor. Do NOT use unapproved accessories or attempt to modify, disassemble or otherwise misuse the Flowtron Hydroven 3 system. Failure to observe this caution could result in injury, or in extreme cases, death. Environmental Protection Incorrect disposal of this equipment and its component parts, particularly batteries or other electrical components, may produce substances that are hazardous to the environment.
-
Page 7: Introduction
If you have any difficulties in setting-up or using the system, contact your local Flowtron Hydroven 3 ArjoHuntleigh sales office, listed at the end of this manual. Intended Use The intended use of this product is to manage the list of clinical conditions detailed in the “Indications”... -
Page 8: Clinical Application
2. Clinical Application Indications Intermittent Pneumatic Compression (IPC) is effective in the treatment of the following clinical conditions, when combined with an individualised monitoring programme: • Edema. • Dependent (including secondary to cerebrovascular incident, pregnancy or paralysis). • Traumatic (post-surgical or injury). •... -
Page 9: Cautions
• Decompensated / severe congestive cardiac failure, pulmonary edema associated with significant limb edema or any condition where an increase of fluid to the heart may be detrimental. • Severe arteriosclerosis or other ischaemic vascular disease. • Active metastatic disease affecting the limb. ... -
Page 10: Clinical Treatment Guide
3. Clinical Treatment Guide An initial pressure setting of 40 mmHg is suggested at the commencement of treatment. It may be necessary to start at a lower level of pressure dependant on the patient’s tolerance. The pressure can be gradually increased over time, until the required pressure is reached. -
Page 11: Garment And Insert Information
4. Garment and Insert Information Garment Description Zip Ring Pull Zip Ring Pull Quick Air Release Bung Tubing Connector Tubing Connector (To Pump) (To Pump) Garment Garment Bung/Connector Bung/Connector For Insert Tubing For Insert Tubing Hydroven 3 Garment Hydroven 1 Garment ... -
Page 12: Applying The Garment
Applying the Garment Quick air release bung Tubing to pump Tubing to pump Leg Garment Arm Garment Before fitting the garment ensure all quick air release bungs are closed, as this will effect the efficiency of the garment. 1. If a larger circumference is required, fit a matching length insert piece before applying to the limb. - Page 13 Leg Garment Arm Garment With Insert Fitted With Insert Fitted 7. Attach the garment tubing to the pump ensuring a “click” is heard from each snap-lock connector. 8. If only one garment is to be used, attach the garment to either port on the pump. The system will automatically identify that only one garment is to be used.
-
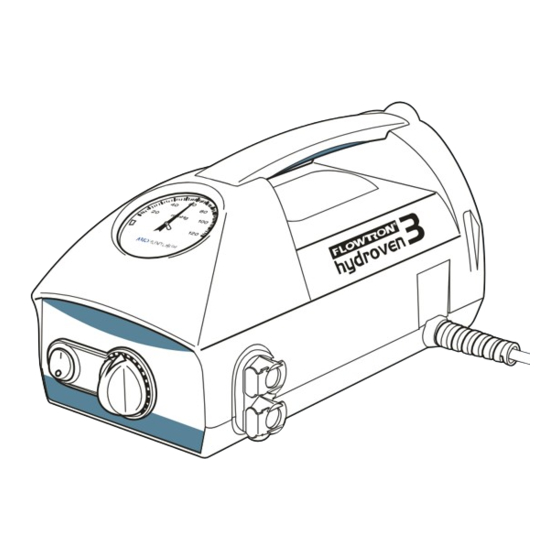
Page 14: Operation
Carry Handle For easy handling of pump If the operation or performance of the pump changes during use, refer to “Troubleshooting” on page 13 of this IFU before calling a service engineer or contacting your local ArjoHuntleigh sales office. -
Page 15: Operation
Caution Do not apply or remove the garment while it is attached to the pump and the pump is in operation, as you may damage the garment zip. Operation The pump should be placed securely on a flat surface. Before starting the pump ensure that the garments are properly applied, the zips are secured and the garment connecting tubes are attached to the pump outlet ports via snap-lock connectors. -
Page 16: Decontamination
Healthcare Facility or the country of use. If you are uncertain, you should seek advice from your local Infection Control Specialist. system should be routinely decontaminated between Flowtron Hydroven 3 patients and at regular intervals while in use; as is good practice for all reusable medical devices. - Page 17 If an alternative disinfectant is selected from the wide variety available, we recommend that suitability for use is confirmed with the chemical supplier prior to use. Wipe down garments using a neutral detergent or soap To Clean and Sterilise powder at 40°C (104°F). Dry thoroughly. Garments After cleaning gas sterilisation is possible, however: •...
-
Page 18: Routine Maintenance
7. Routine Maintenance Flowtron Hydroven 3 System The equipment has been designed to be maintenance- Maintenance free between service periods. will make available on request service Servicing ArjoHuntleigh manuals, component parts lists and other information necessary for trained personnel to repair ArjoHuntleigh the system. -
Page 19: Troubleshooting
Check garment. Replace if defective. Service Returns If for any reason your is being Flowtron Hydroven 3 returned, please: 1. Clean the product following the instructions in section “Decontamination” on page 10. 2. Enclose a covering letter explaining the reasons for the return, with documentation (including an appropriate decontamination certificate if possible). -
Page 20: Accessories
9. Accessories To order garments, please contact your distributor quoting the appropriate codes as shown below: Refer to diagrams on page 5 of this manual. Garments HYDROVEN 1 LEG GARMENTS Order Code Type Length (L) Circumference (W) 5101L50 Half Leg 19.7"... -
Page 21: Inserts
Inserts HYDROVEN INSERT PIECES (To Fit Hydroven 1 and Hydroven 3 Garments) Order Code Type Length (L) Added Circumference Added Circumference Wide End Narrow End 510LI50 Half Leg 19.7" (50cm) 7.5" (19cm) 5.5" (14cm) 510LI66 Full Leg 26" (66cm) 7.5" (19cm) 5.5"... -
Page 22: Technical Specification
10. Technical Specification Pump Model: Flowtron Hydroven 3 Part Numbers: 510003 Pressure Range: 30 - 100 mmHg ± 5% Supply Voltage: 120 V Supply Frequency: 60 Hz Power Input: 14 VA Size: 10.6 x 5.9 x 4.1” (270 x 150 x 105 mm) Weight: 5.3 lb (2.4 kg) -
Page 23: Cleaning Symbols
Symbols Refer to this document Power (Instructions for Use) for Do not dispose of in Disconnects from the a description of the domestic refuse (Off) mains supply product classification (2nd Edition). Refer to this document Power (Instructions for Use) for Connects to the Double Insulated a description of the... - Page 24 Guidance and manufacturer’s declaration - electromagnetic emissions The pump is intended for use in the electromagnetic environment specified below. The customer or the user of the pump should assure that it is used in such an environment. Emissions Test Compliance Electromagnetic environment - guidance RF emissions Group 1...
- Page 25 Guidance and manufacturer’s declaration - electromagnetic immunity The pump is intended for use in the electromagnetic environment specified below. The customer or the user of the pump should assure that it is used in such an environment. Immunity Test IEC 60601 Test Compliance Electromagnetic environment - guidance Level...
- Page 27 ÖSTERREICH Τηλ: 21 0724 36 68 ArjoHuntleigh GmbH Φάξ: 21 0721 55 53 POLSKA Dörrstrasse 85 ArjoHuntleigh Polska Sp. z o.o. AT-6020 INNSBRUCK ESPAÑA ul. Ks Piotra Wawrzyniaka 2 Tel: +43 (0) 512 204 160 0 ArjoHuntleigh Ibérica S.L. PL 62-052 KOMORNIKI (Poznan) Fax: +43 (0) 512 204 160 75 Ctra.
- Page 28 We operate under the three brands of ArjoHuntleigh, GETINGE and MAQUET. ArjoHuntleigh focuses on patient mobility and wound management solutions. GETINGE provides solutions for infection control within healthcare and contamination prevention within life sciences.


Need help?
Do you have a question about the FLOWTRON HYDROVEN 3 and is the answer not in the manual?
Questions and answers