Summarization of Contents
Olympus EndoTherapy Device Identification
Rotatable Clip Fixing Device Models
Lists the various models of the Rotatable Clip Fixing Device and associated clips.
Understanding Device Symbols
Instructional and Identification Symbols
Explains symbols for instructions, use, sterilization, lot numbers, and manufacturer information.
Important Safety Information
Intended Use and Indications for Use
Specifies the intended use of the instrument for endoscopic clip placement in the GI tract.
Manual Guidance and Compatibility
Instruction Manual and User Qualifications
Emphasizes reading the manual and defines operator qualifications for safe use.
Instrument Compatibility Checks
Advises checking compatibility with ancillary equipment to prevent damage.
Handling and Maintenance Precautions
Reprocessing and Storage Guidelines
Instructions on reprocessing and storing the instrument before and after use.
Prohibition of Repair and Modification
Prohibits user disassembly, modification, or repair of the instrument.
Safety Communication Standards
Signal Words and General Warnings
Defines signal words (WARNING, CAUTION, NOTE) and provides general safety warnings.
Specific Usage Cautions and Notes
Details specific warnings and notes regarding hemostasis, MRI, lesion types, and re-bleeding.
Chapter 1: Package Contents Verification
Checking Package Contents
Instructions to match and inspect all items in the package for damage or missing components.
Chapter 2: Instrument Details and Specifications
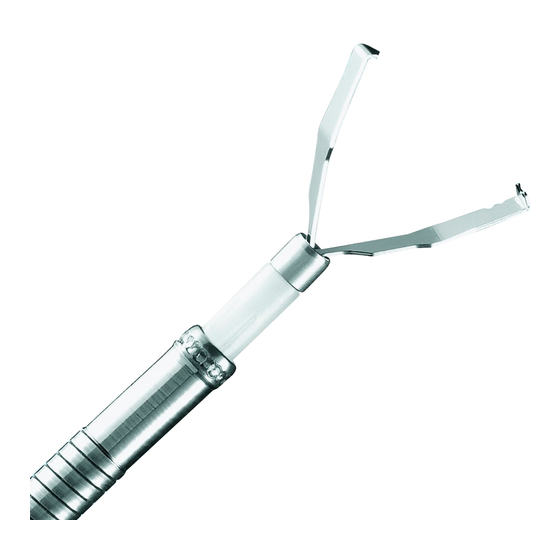
Nomenclature and Functions of the Device
Describes the parts and functions of the Rotatable Clip Fixing Device.
Clip Specifications and Usage Warnings
Details clip types and provides warnings on using compatible products.
Operating Environment and Model Specs (Part 1)
Outlines operating environment and specifications for HX-110LR and HX-110QR models.
Model Specifications (Part 2)
Details specifications for HX-110UR, HX-610-090, and HX-610-135 models.
Model Specifications (Part 3)
Details specifications for HX-610-090L and HX-610-135S models.
Model Specifications (Part 4) and Compliance
Details specifications for HX-610-090SC, HX-610-090S models and medical device compliance.
Chapter 3: Preparation, Inspection, and Operation
Pre-use Warnings and Procedures
Covers warnings on clip expiration, inspection, damage, and initial instrument reprocessing.
Preparation Steps
Details preparation steps including equipment, PPE, spares, and emergency equipment.
Inspection Procedures
Instructions for inspecting the instrument and sterile package for any damage or irregularities.
Operation Inspection
Procedures to check if the instrument operates smoothly and as intended before use.
Basic Operation Guidelines
Outlines basic operation procedures and necessary precautions for the operator.
Attaching the Clip to the Device
Step-by-step instructions and warnings for properly attaching a clip to the instrument.
Insertion into the Endoscope
Detailed instructions and warnings for safely inserting the instrument into the endoscope.
Clipping Tissue Procedure
Procedures and warnings for clipping tissue with the instrument, including deployment and orientation.
Chapter 4: Emergency Procedures
Emergency Treatment for Detachment Issues
Procedures to follow if the clip cannot be detached from the instrument, including warnings.
Detaching and Removing Clip Components
Instructions for detaching the clip connector and removing aspirated components from the endoscope.
Chapter 5: Instrument Reprocessing
General Reprocessing Policy and Warnings
Covers general guidelines, responsibilities, and warnings related to reprocessing endoscopic equipment.
Required Reprocessing Equipment
Lists the necessary equipment for reprocessing, including basins, cleaners, and lubricants.
Cleaning Procedures
Detailed instructions for cleaning the instrument, including immersion, ultrasonic cleaning, and rinsing.
Lubrication Process
Instructions for lubricating the instrument after cleaning to ensure smooth operation.
Sterilization and Packaging
Procedures for sealing the instrument package and preparing for sterilization.
Steam Sterilization (Autoclaving) Guidelines
Guidelines for steam sterilization, including package placement, drying, and monitoring.
Chapter 6: Storage Guidelines
Inspection Before Storage
How to inspect the sterile package for damage before storing the instrument.
Proper Storage Conditions
Guidelines for storing the instrument in its sterile package at room temperature in a clean, dry environment.















Need help?
Do you have a question about the EndoTherapy HX-110UR and is the answer not in the manual?
Questions and answers