Advertisement
Available languages
Available languages
DE/GB
GB Safety instructions
Instruction manual
GB
Blood Pressure Monitor
BU 570 connect
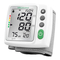
Unit and LC-Display
•
•
•
1
•
2
REF
Reference number
•
•
•
3
LOT
Batch number
•
REF
Reference number
•
REF
Reference number
•
4
I
56 7
ON
LOT
•
Batch number
LOT
Batch number
io
1
•
CAUTION /
I
OFF
注意!查阅随机文件
ON
•
I
u
•
8
/
(b)
2-3 cm
•
ON
z
Type BF applied part /
(a)
Dispose of packaging in an
•
BF型应用设备
environmentally friendly manner
t
•
OFF
•
9
2
Read the instructions for use /
•
r
OFF
请阅读说明书
(c)
0
•
Dispose of packaging in an
•
Device in protection class 2 /
environmentally friendly manner
•
Importer
3
Dispose of packaging in an
二类设备
•
environmentally friendly manner
e
w
q
•
•
Manufacturer / 制造商
•
Importer
Explanation of symbols
•
Importer
IMPORTANT
Keep dry / 保持干燥 / 怕雨
•
Follow the instructions for use!
Non-observance of these instructions can result in seri-
•
•
ous injury or damage to the device.
WEEE
•
WARNING
These warning notes must be observed to prevent any
•
injury to the user.
•
SN
Serial number
CAUTION
•
These notes must be observed to prevent any damage
Storage/Transport
to the device.
•
Permissible storage and transport
temperature and humidity
•
NOTE
•
These notes give you useful additional information on
Operation
the installation or operation.
Permissible operating temperature
Storage/Transport
•
and humidity
70°C
95%
1060hPa
e
•
Device classification: type BF applied part
-25°C
10%
700hPa
•
Medical device
LOT number
Medical device
Operating
•
Storage/Transport
SN
1060hPa
Serial number
40°C
95%
Ambient pressure
BATTERY SAFETY INFORMATION
1060hPa
70°C
limitation
95%
•
10°C
700hPa
Storage/Transport
Distributor
10%
Direct current
•
10%
700hPa
-25°C
1060hPa
70°C
95%
•
Manufacturer
Temperature range
•
Operating
10%
700hPa
-25°C
Date of manufacture
•
40°C
95%
1060hPa
Operating
•
Date of manufacture
Humidity range
•
700hPa
10°C
10%
40°C
95%
1060hPa
Do not use outdoors
•
700hPa
10°C
Recycling symbols/codes:
10%
•
21
21
(indoor use only)
These are used to provide information about
•
•
the material and its proper use and
20
recycling.
PAP
Single patient multiple use
a GmbH
Unit and LC-Display
urz-Str.2, 41460 NEUSS
NY
Intended use
edisana.de
1
• This fully automatic electronic blood pressure monitor is
PA
4
intended for measuring the blood pressure at home. It is a non-
中国GCC血压计计量证号
7
invasive blood pressure measurement system to measure the
2020F213-44
0
diastolic and systolic blood pressure and pulse of an adult using
e
an oscillometrique technique by means of a cuff, which needs to
t
be fitted on the upper arm. The useable cuff circumference is 22
i
to 42 cm.
• The device is intended for adult indoor use only.
Scope of supply
Please check first of all that the device is complete and is not damaged in any way. In case of doubt, do not use it and contact
Contraindications
your dealer or your service centre.
• The device is not suitable for use on pregnant women and
The following parts are included:
patients with implanted, electronical devices (e.g. pacemaker or
• 1 medisana blood pressure monitor BU 570 connect
defibrillator).
• 4 batteries (type AAA, LR03) 1.5V
• 1 instruction manual and EMV leaflet
0297
Please contact your supplier if you notice any transport damage on unpacking the unit.
Read the instruction manual carefully before using this device, es-
pecially the safety instructions, and keep the instruction manual for
future use. Should you give this device to another person, it is vital
that you also pass on these instructions for use.
The unit is intended only for private use in the home.
Only use the unit for its intended purpose in accordance with the instructions for use. Warranty claims become void if the
unit is misused.
The patient is an intended operator. The patient can measure data and change batteries under normal circumstances and
maintain the device and its accessories according to the user manual.
This blood pressure monitor is intended for adult use. The device is not suitable for measuring the blood pressure of children
or infants. Ask your doctor before using it on older children.
Keep the unit out of reach of infants, young children or pets to avoid inhalation or swallowing of small parts.
The device has sensitive components. Be careful and ensure that the operating and storage conditions are met.
The device is not suitable for use on pregnant women, patients with implanted, electronical devices (e.g. pacemaker or
defibrillator), patients with pre-eclampsia, premature ventricular beats, atrial fibrillation, peripheral, arterial disease and
patients undergoing intravascular therapy or arterio-venous shunt or people who received a mastectomy. Please consult
your doctor prior to using the unit if you suffer from illnesses.
The device is not intended for patient transport outside a healthcare facility.
This device is intended for non-invasive measuring and monitoring of arterial blood pressure on the upper arm. It is not
intended for use on extremities other than the upper arm or for functions other than obtaining a blood pressure measurement.
Do not take any therapeutic measures on the basis of a self measurement. Never alter the dose of a medicine prescribed
by a doctor. If you are taking medication,consult your physician to determine the most appropriate time to measure your
blood pressure. Contact your doctor if you have any further questions about blood pressure measurement with this device.
Irregularity of pulse or arrhythmia can lead to difficulties in recording a correct reading when measurements are taken using
oscillometric blood pressure devices. This device is electronically equipped to detect arrhythmia which it indicates with the
icon
q
. In this case, contact your physician.
Like on any other oscillometric blood pressure measurement devices, certain medical conditions can affect the measurement
REF
accuracy, among others: cardiac arrhythmias, low blood pressure, circulatory disorders, states of shock, diabetes, pregnancy,
Reference number
pre-eclampsy etc. Always consult a physician before you use the device.
Pay attention during use and storage, that cable and air hose will not cause strangulation.
Never bend the air hose during use as this may lead to injuries. Also never squeeze or block the air hose in any way.
Do not take measurements, if any other equipment is applied to the same limb for monitoring. This could lead to disturbances
LOT
Batch number
or failures.
Too frequent and consecutive measurements could cause disturbances in blood circulation and injuries.
This device cannot be used with surgical equipment at the same time.
Do not apply the cuff over a wound, otherwise it can cause further injury.
I
On the rare occasion of a fault causing the cuff to remain fully inflated during measurement, open the cuff immediately.
ON
Prolonged high pressure (cuff pressure >300 mmHg or constant pressure >15 mmHg for more than 3 minutes) applied to
the arm may lead to an ecchymosis.
To verify the calibration of the device, contact the customer service.
This device is not suitable for continous monitoring during medical emergencies or operations - risk of injuries!
OFF
Do not use the equipment where flammable gas (such as anaesthetic gas, oxygen or hydrogen) are present.
Before use, make sure the device functions safely and is in proper working condition. Do not service or maintain while the
device is in use.
Check the device for damage. The use of a damaged device can result in injury, incorrect readings or serious hazards.
Dispose of packaging in an
The unit may not be operated in the vicinity of appliances that emit strong electric radiation such as radio transmitters or
environmentally friendly manner
mobile phones. This may affect the proper functioning (see "electromagnetic compatibility").
The device and its components are suitable for use within the patient environment. If you are allergic to polyester, nylon or
plastic, please don't use this device.
If you experience discomfort during a measurement, such as pain in the upper arm or other complaints, press the START/
STOP button
7
to release the air immediately from the cuff. Loosen the cuff and remove it from your arm.
If the cuff pressure reaches 300 mmHg, the unit will automatically deflate. Should the cuff not deflate when pressure reaches
Importer
300 mmHg, detach the cuff from the arm and press the START/STOP button
7
to stop inflation.
Do not wash the cuff in a washing machine or dishwasher!
The service life of the cuff may vary by the frequency of cleaning, skin condition, and storage state. The typical service life
is 10,000 times.
It is recommended that the performance should be checked every 2 years and after maintenance and repair. Contact the
customer service to learn more.
Do not touch output of batteries and the patient simultaneously.
Please do not attempt to repair the unit yourself in the event of malfunctions. Only have repairs carried out by authorised
service centres.
Please ensure that the unit is kept away from the reach of children and pets. The swallowing of small part like packaging bag,
battery, battery cover and so on may cause suffocation.
Only use original additional or spare parts from the manufacturer. Other parts could damage the device or could lead to
personal injury.
Please report to medisana if any unexpected operation or events occur.
The construction of the device possibly allows an inadvertently connection to intravascular fluid systems, allowing air to be
pumped into a blood vessel - risk of injury!
Only use the device under the conditions mentioned in the technical details. Otherwise, the lifespan and performance of the
device could suffer.
At least 30 min required for ME equipment to warm from the minimum storage temperature between uses until it is ready
for the intended use.
At least 30 min required for ME equipment to cool from the maximum storage temperature between uses until it is ready for
the intended use.
Storage/Transport
If the unit is not going to be used for a long period, please remove the batteries.
70°C
95%
1060hPa
-25°C
10%
700hPa
Do not disassemble batteries!
Operating
Renew the batteries when the battery icon appears on the display.
1060hPa
Remove discharged batteries from the device immediately, because they can leak and damage the device!
40°C
95%
Increased risk of leakage, avoid contact with skin, eyes and mucous membranes! If battery acid comes in contact with
10°C
700hPa
any of these parts, rinse the affected area with copious amounts of fresh water and seek medical attention immediately!
10%
If a battery has been swallowed seek medical attention immediately!
Replace all of the batteries simultaneously!
Only replace with batteries of the same type, never use different types of batteries together or used batteries with new
ones.
Insert the batteries correctly, observing the polarity!
Keep batteries out of children's reach!
Do not attempt to recharge batteries, do not short-circuit and do not throw into a fire! There is a danger of explosion!
Do not throw used batteries into the household refuse; put them in a hazardous waste container or take them to a battery
collection point, at the shop where they were purchased!
Cuff
Air hose
2
3
Battery compartment (on backside)
5
MEM-button
6
START/STOP-button
8
Systolic pressure
9
Pulse rate
Pulse display
q
w
Movement detected
Bluetooth symbol
r
Average value display ("AVG")
User memory 2
z
u
Blood Pressure Indicator (green - yellow - orange - red)
o
• 1 cuff with air tube
• 1 storage pouch
WARNING - Please ensure that the polythene packing is kept away from the reach of
children! Risk of suffocation!
How is blood pressure measured?
This medisana blood pressure monitor is a device, which is used to measure blood pressure at the upper arm. The
measurement is carried out by a microprocessor - already during the inflating process of the cuff. The device detects the systole
and the measurement is completed earlier than using conventional measurement methods. As a result, an unnecessarily
excessive inflation pressure in the cuff is prevented. Additionally, your device is able to recognize unregular heartbeat (so-
called arrhythmia), which may affect the measurements. In case an arrhythmia has been deteced, the respective symbol will
appear in the display of the unit.
Blood pressure classification
systolic mmHg
diastolic mmHg
≥ 180
≥ 110
160 - 179
100 - 109
140 - 159
90 - 99
130 - 139
85 - 89
120 - 129
80 - 84
< 120
< 80
WARNING - Blood pressure that is too low represents just as great a health risk as blood
pressure that is too high! Fits of dizziness may lead to dangerous situations arising (e.g. on
stairs or in traffic)!
Influencing and evaluating readings
•
Measure your blood pressure several times, then record and compare the results. Do not draw any conclusions from a single
reading.
•
Your blood pressure readings should always be evaluated by a doctor who is also familiar with your personal medical history.
When using the unit regularly and recording the values for your doctor, you should visit the doctor from time to time to keep
him updated.
•
When taking readings, remember that the daily values are influenced by several factors. Smoking, consumption of alcohol,
drugs and physical exertion influence the measured values in various ways.
•
Measure your blood pressure before meals.
•
Before taking readings, allow yourself at least five minutes rest.
•
If the systolic and diastolic readings seem unusual (too high or too low) on several occasions, despite correct use of the unit,
please inform your doctor. This also applies to the rare occasions when an irregular or very weak pulse prevents you from
taking readings.
Getting started
Inserting/changing batteries
You must insert the batteries provided before you can use your unit. The lid of the battery compartment
underside of the unit. Open it, remove it and insert the 4 x 1,5 V batteries, type AAA / LR03 supplied. Ensure correct polarity
when inserting the batteries (as marked inside the battery compartment). Close the battery compartment. Replace the batteries
if the change battery symbol
o
and "Lo" appears on the display or if nothing appears on the display after the unit has been
switched on.
Settings
Setting date and time
When unit is switched off, press SET
6
. The time will be displayed. Now press and hold the SET button
starts to flash for setting. Keep pressing the MEM button
year. Afterwards, you can proceed with the month/date setting, followed by the time format setting (12 or 24 hours), as well as
hours and minutes. Adjust the corresponding figures accordingly. When finished, "dOnE" appears on the display along with the
adjusted values. Afterwards, the device will switch off.
User memory setting
The MEDISANA blood pressure monitor features 2 separate memories, each with a capacity of 120 memory slots. By pressing
and holding the MEM button
5
when the device is switched off, you may choose between user 1
shows the current selection. Confirm your selection with the SET button
Fitting the cuff
1. Connect the air hose to the connector
3
on the left side of the device.
2. Slide the open end of the cuff through the metal bracket so that the Velcro fastener is on the outside and it becomes a
cylindrical form (Fig.1). Slide the cuff over your left upper arm.
3. Position the air hose in the middle of your arm in line with your middle finger (Fig.2) (a). The lower edge of the cuff should
be 2 - 3 cm above the crease of the elbow. (b). Pull the cuff tight and close the Velcro fastener (c).
4. Measure the blood pressure on your bare arm.
5. Only position the cuff on the right arm if it cannot be used on the left arm. Always carry out measurements on the same arm.
6. Correct measuring position for sitting (Fig.3).
Taking a blood pressure measurement
After the cuff has been appropriately positioned,the measurement can begin.
1. Switch the unit on by pressing the START/STOP button
2. All display characters are shown (display test). This test can be used to check that the display is indicating properly and in
full. The unit is ready for measurement and „0" appears.
3. The device then automatically inflates the cuff slowly in order to measure your blood pressure. The rising pressure in the
cuff is shown on the display.
4. When the device detects the signal, the heart symbol on the display starts to flash. If a result is determined, the device
slowly will release the air from the cuff. The systolic and diastolic blood pressure and the pulse value with the time value
appear on the display.
5. The blood pressure indicator
i
flashes next to the relevant coloured bar depending on the blood pressure classification.
6. If the unit has detected an irregular heartbeat, the display
7. The readings are automatically saved in the selected memory (
8. The measurement results will automatically be transferred via Bluetooth to receive-ready devices. During this process, the
Bluetooth-symbol
r
will appear. If the transfer was not successful, the Bluetooth-symbol
switch off. If the transfer was successful, the Bluetooth-symbol will NOT flash.
9. Switch off the device using the START/STOP button
7
Transfer via Bluetooth
This device offers the possibility to transfer your measured values via Bluetooth to the VitaDock
allows the evaluation, storage and synchronisation of your data between multiple iOS- and Android-devices to have access
to it anytime and anywhere. You may share your results with your friends or your doctor. Therefore you need a free user
Connector for the hose
account, which you can create on the website www.vitadock.com. For Android or iOS mobile devices, you may download the
SET-button
respective apps. You will find a detailed instruction for how to install and use the software on the mentioned website. After each
Diastolic pressure
measurement an automatic transfer of the values will take place (provided that Bluetooth is activated and configurated on the
receiving device).
Time/Date
WARNING
User memory 1
• Keep the device at least 20 centimeters away from the human body (especially the head)
Battery symbol
when the Bluetooth data transmission is proceeding after measurement.
• The distance between the device and the receiver should be between 1 meter to 10 meters
to avoid possible disturbances.
• To avoid interference with other electronic devices, these should be kept at a minimum
distance away from the equipment. The distanced is calculated from the 80MHz to 5.8GHz
column of Table 4 and Table 9 of IEC 60601-1-2:2014, as appropriate.
Bluetooth Module no.: LS8261
RF Frequency range: 2400 MHz - 2483.5 MHz; Output power range: ≤8dBm;
Supply voltage: 1.9-3.6V; Transmitting distance: 10 m
Displaying stored results
This unit features 2 separate memories, each with a capacity of 120 memory slots. Results are automatically stored in the
memory selected by the user. Press the MEM button
values of the last 3 measurements appear on the display (if already 3 measurements have been stored for the respective user.
Press the MEM button
button
6
displays the respective values measured in other measurements. Memory recall mode can be exited at any time by
pressing the START/STOP button
have been stored in the memory and a new value is saved.
Deleting stored values
Enter the memory recall mode as described above and press and hold the MEM button
will be displayed. Now press and hold the START/STOP button
device will switch itself off. To exit the deletion mode, you may press the START/STOP button
Error messages and error remedying
Display
Bloodpressure indicator
No display
i
serious hypertension
red
medium hypertension
orange
+ Lo
mild hypertension
yellow
high-normal blood pressure
green
normal blood pressure
green
E01
optimal blood pressure
green
E02
E03
E04
EExx
out
Cleaning and maintenance
Remove the batteries before cleaning the unit. Clean the unit using a soft cloth lightly moistened with a mild soapy solution.
Never use abrasive cleaning agents, alcohol, naphta, thinner or gasoline etc. Never immerse the unit or any component in
water. Be cautious not to get any moisture in the main unit. Do not wet the cuff or attempt to clean the cuff with water. Using a
dry cloth, gently wipe away any excess moisture that may remain on the cuff. Lay the cuff flat in an unrolled position and allow
the cuff to air dry. Do not expose the unit to direct sunlight; protect it against dirt and moisture. Do not expose the unit to extreme
is located on the
4
hot or cold temperatures. Keep the device in the storage case when not in use. Store the unit in a clean and dry location.
Disposal
This product must not be disposed of together with domestic waste. All users are obliged to hand in all
electrical or electronic devices, regardless of whether or not they contain toxic substances, at a municipal or
commercial collection point so that they can be disposed of in an environmentally acceptable manner.
Remove the batteries before disposing of the device/unit. Do not dispose of old batteries with your household
waste, but at a battery collection station at a recycling site or in a shop. Consult your local authority or your
supplier for information about disposal.
6
, until the year figure
until the actual year figure appears. Press SET
to confirm the
5
6
Guidelines / Standards
This blood pressure monitor meets the requirements of the EU standard for noninvasive blood pressure monitors. It is certified
in accordance with EC Guidelines and carries the CE symbol (conformity symbol) "CE 0297". The blood pressure monitor
corresponds to European standards EN 60601-1, EN 60601-1-2, EN 60601-1-6, EN 60601-1-11, EN 1060-3, EN 81060-1 and
EN 81060-2. The specifications of EU Guideline "93/42/EEC of the Council Directive dated 14 June 1993 concerning medical
devices" are met and likewise those of the RED 2014/53/EC. You can request the complete Conformity Declaration from
medisana GmbH, Carl-Schurz-Str. 2, 41460 Neuss, Germany, or you can also download it from the medisana homepage
u
and user 2
z
. The display
(www.medisana.de). Electromagnetic compatibility: (see attached leaflet)
6
.
Technical specifications
Name and description:
Software version:
Display system / Memory slots:
Measuring method / Mode of operation:
Power supply:
Rated cuff pressure:
Measurement pressure:
Pulse measuring range:
Maximum error tolerance for static pressure:
Maximum error tolerance for pulse rate:
Pressure generation:
7
.
Deflation:
Protection marking against particles and water:
Operating conditions:
Storage conditions:
Dimensions (L x W x H):
Cuff:
Weight (main unit):
Item number:
q
appears additionally
EAN number:
z
or
u
).
Acessories:
r
will flash and the device will
In accordance with our policy of continual product improvement, we reserve the right to make technical and visual
.
Warranty and repair terms
app. The VitaDock
app
®
®
Your statutory warranty rights are not restricted by our guarantee below. Please contact your supplier or the service centre in
case of a claim under the warranty. If you have to return the unit, please enclose a copy of your receipt and state what the defect is.
The following warranty terms apply:
1. The warranty period for medisana products is 3 years from date of purchase. In case of a warranty claim, the date of
purchase has to be proven by means of the sales receipt or invoice.
2. Defects in material or workmanship will be removed free of charge within the warranty period.
3. Repairs under warranty do not extend the warranty period either for the unit or for the replacement parts.
4. The following is excluded under the warranty:
a. All damage which has arisen due to improper treatment, e.g. nonobservance of the user instructions.
b. All damage which is due to repairs or tampering by the customer or unauthorised third parties.
c. Damage which has arisen during transport from the manufacturer to the consumer or during transport to the service centre.
d. Accessories which are subject to normal wear and tear.
5. Liability for direct or indirect consequential losses caused by the unit are excluded even if the damage to the unit is accepted
as a warranty claim.
medisana GmbH
Carl-Schurz-Str. 2
41460 NEUSS, GERMANY
The service centre address is shown on the attached leaflet.
5
when power off, to call up the measured values stored. The average
5
again to call up the last measurement value. Repeatedly pressing the MEM button
7
, which will also switch the unit off. The oldest value is deleted if 120 measured values
5
until „dEL ALL" and the user (1 or 2)
to delete all values (" dEL donE" and the use appear). The
7
7
at any time.
Cause
Solution
Batteries may be exhausted or inserted incorrectly.
Insert new batteries.
Check if the power connection has been
Insert the batteries according to the instructions
established properly.
given.
Weak batteries
Batteries are weak or empty.
Replace all 4 batteries by new ones (1.5V, type AAA
/ LR03).
Cuff not properly fitted
Wrap the cuff properly.
Remeasure in correct way.
Movement or speaking
Rest 30 minutes and remeasure in correct way.
during measurement
Do not speak or move during the measurement.
Pulse cannot be detected
Wrap the cuff properly.
Loosen the clothing on the arm.
Remeasure in correct way.
Measurement failed
Wrap the cuff properly.
Remeasure in correct way.
Calibration error ("xx" can be a figure,
Wrap the cuff properly.
e.g. 01, 02 etc.)
Remeasure in correct way.
Out of measurement
Relax for a moment. Refasten the cuff and measure
range
again. If the problem persists, contact your physician.
medisana Blood pressure monitor BU 570 connect
A01
Digital display / 2 x 120
Oscillometric / Continuous operation
6 V
, 4 x 1,5 V batteries AAA LR03, internally powered
0 - 299 mmHg (0 - 39.9 kPa)
SYS: 60-230mmHg (8-30.7kPa); DIA: 40-130mmHg (5.3-17.3kPa)
40 – 199 beats per minute
± 3 mmHg
± 5 % of the value
Automatic with pump
Automatic
IP21
+5°C to +40°C, 15 to 90 % relative humidity, non-condensing,
700-1060 hPa atmospheric pressure
-20°C to +60°C, relative humidity ≤ 93 %
approx.130 x 93 x 32.5 mm
22 - 42 cm for adults
approx. 185 g without batteries
51204 (BU 570 black); 51203 (BU 570 white)
40 15588 51204 9; 40 15588 51203 2
Spare cuff : Art. No. 51299 / EAN 40 15588 51299 5
changes without notice.
The current version of this instruction manual can be found under www.medisana.com
5
or the SET
Advertisement