Summary of Contents for MESI mTABLET TBI
- Page 1 Instructions for Use MESI mTABLET TBI Toe-Brachial Index MTABSYSTBI ISO 9001 Q-1664 ISO 13485 M-049...
- Page 2 DISTRIBUTOR INFORMATION CONTACT INFORMATION Address MESI, development of medical devices, Ltd Leskoškova cesta 11a SI-1000 Ljubljana Slovenia, European Union Telephone +386 (0)1 620 34 87 E-mail info@mesimedical.com Website www.mesimedical.com...
- Page 3 Instructions for Use MESI mTABLET TBI Toe-Brachial Index ISO 9001 Q-1664 ISO 13485 M-049...
-
Page 4: Table Of Contents
4 QUICK MEASURING GUIDE 4.1 PREPARATION FOR MEASUREMENT 4.1.1 Pairing with the MESI mTABLET UNIT 4.1.2 Assembly of the MESI TOE BLOOD PRESSURE UNIT 4.1.3 Assembly of the MESI TUBELESS CUFF UNIT 4.1.4 Patient Preparation 4.1.5 Performing a TBI Measurement 4.2 RESULTS... - Page 5 5.3 PERFORMING A TBI MEASUREMENT 5.3.1 Cuff Placement 5.3.2 Performing a TBI Measurement 5.4 REVIEWING A TBI MEASUREMENT 5.4.1 MESI mTABLET Results Screen 5.4.1.1 Navigation Area 5.4.1.2 Measurement Information 5.4.1.3 PPG Pulse Waveform Recordings 5.4.1.4 Oscillation Graphs and Pulse Waveform Recordings 5.4.1.5 Patient Measurement History...
- Page 6 7.3 MAINTENANCE 7.4 DEVICE FUNCTIONALITY 8 ERRORS 9 TROUBLESHOOTING 10 WARRANTY INFORMATION 11 STANDARD COMPLIANCE 11.1 MANUFACTURER DECLARATION ON EMC 11.2 ESSENTIAL PERFORMANCE 12 IMPORTANT LABELS NOTES...
-
Page 7: Safety And Legal Recommendations
The MESI mTABLET TBI users must be adequately trained in use of the device. Users must carefully read the entire Instructions for Use prior to initial use of the device and follow the Instructions for Use for the... -
Page 8: Product Description
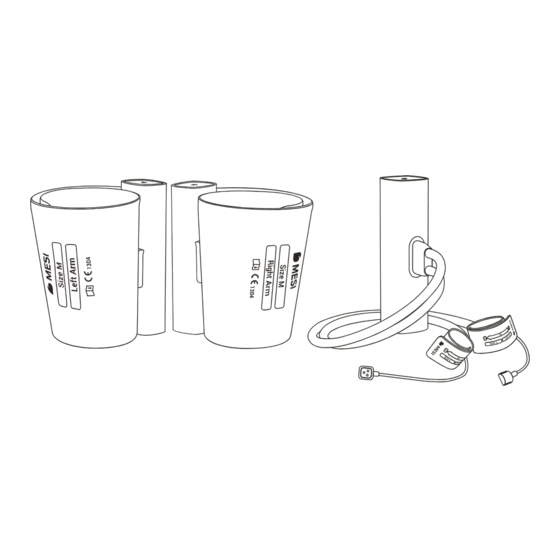
PRODUCT DESCRIPTION The MESI TBI MODULE is a wireless Toe-Brachial Index module designed for the MESI mTABLET TBI System. Toe-Brachial Index measurements and other parameters are displayed on the MESI mTABLET. PRODUCT DESCRIPTION 2.1 WHAT IS IN THE PACKAGE? The MESI TBI MODULE package includes the following equipment: •... -
Page 9: Accessories
Contact your local distributor for more information about different cuff sizes and other accessories. MESI mTABLET TBI users must be adequately trained in use of the device. Users must carefully read and follow the entire Instructions for Use prior to initial use of the device. - Page 10 The device is recharged through an AC/DC power supply, however, the MESI mTABLET TBI is not intended to be used while connected to mains electricity.
-
Page 11: Technical Specifications
TECHNICAL SPECIFICATIONS The technical information regarding the MESI TOE BLOOD PRESSURE UNIT and the MESI TUBELESS CUFF UNIT provided within the package is as follows: TECHNICAL SPECIFICA- TIONS 3.1 MESI TOE BLOOD PRESSURE UNIT (TBPMD) 3.1.1 40 mm (1.57 inches) -
Page 12: Power & Battery
TECHNICAL SPECIFICATIONS 3.2.2 Battery Type Rechargeable lithium-polymer battery POWER & (LP602248) BATTERY Capacity 1240 mAh AC/DC Adaptor FW8030M/05 (FRIWO FOX30-XM) Input 100-240V AC/50-60Hz/600-300mA Output 5V DC/5.0A Examinations per > 200 Battery Charge 3.2.3 Medium-sized Cuffs Tubeless cuff set - BP - Medium CUFF SIZES –... -
Page 13: Measurement Specifications
TECHNICAL SPECIFICATIONS Measurements Using Oscillometry, Volume Plethysmography 3.3.3 and Photoplethysmography: MEASUREMENT SPECIFICATIONS • Toe-Brachial Pressure Index • Systolic blood pressure (arms and toes) • Diastolic blood pressure (arms) • Heart rate Measurement Range: • Arm pressure: 0 to 299 mmHg •... -
Page 14: Quick Measuring Guide
Before using the device for the first time, read the Instructions for Use carefully and follow the recommendations and suggestions. This chapter only includes short instructions for the use of the MESI mTABLET TBI. See Chapter 5 DETAILED INSTRUCTIONS for a detailed description of the device's individual QUICK functions. -
Page 15: Assembly Of The Mesi Toe Blood Pressure Unit
4.1.2 ASSEMBLY during storage and transportation. Prior to initial use connect the OF THE MESI toe blood pressure cable to the port on the MESI TOE BLOOD TOE BLOOD PRESSURE UNIT, as shown on the image below. PRESSURE UNIT After ensuring that the cable is securely connected, attach the digit cuffs into corresponding cuff connector as shown on the image below. -
Page 16: Patient Preparation
• Make sure that the vertical indicator line falls within area marked with the OK sign. If not, select an appropriately sized cuff. NOTE The arm cuffs can be connected to either of the MESI TUBELESS CUFF UNITS. The colour, size and positioning will be automatically detected by the MESI TUBELESS CUFF UNIT. -
Page 17: Performinga Tbi Measurement
On the MESI mTABLET UNIT select existing After selecting the patient, please select the patient (1) or add a new one (2). TBI measurement in the application menu. NOTE For additional information see the MESI mTABLET Instruction Manual – Chapter 8.1 PATIENT SELECTION. - Page 18 QUICK MEASURING GUIDE Options Select type of measurement: Step 3 4-cuff SmartArm 3-cuff arm selection Options Select default arm to measure TBI: When starting the TBI applica- Select type of measurement: Left arm Right arm 4-cuff SmartArm 3-cuff arm selection tion for the first time the default At 3-cuff manual measurement provider chooses an arm to measure TBI.
- Page 19 QUICK MEASURING GUIDE Step 5 Wrap the digit cuffs around the base of the appropriate big toe. Make sure that the cuffs are not too tight to prevent any residual pressure. Place the PPG probe against the skin on the fleshy part of the appropriate big toe and secure it with the enclosed fastener strap or medical tape.
-
Page 20: Results
INSTRUCTIONS 5.1 FIRST-TIME USE MTABSYSTBI is a wireless system intended for measuring Toe-Brachial 5.1.1 Index (TBI). System is comprised of MESI mTABLET UNIT, MESI TOE BASIC FUNCTIONALITIES BLOOD PRESSURE UNIT, 2x MESI TUBELESS CUFF UNIT and MESI LARGE CHARGING PLATE (CS4SYS). -
Page 21: Ac/Dc Power Supply And Battery
When setting up the MESI TOE BLOOD PRESSURE UNIT and the 5.1.3 MESI TUBELESS CUFF UNIT for the first time it needs to be activated ACTIVATION out of the shipping mode. The device will not respond until it is placed on the MESI LARGE CHARGING PLATE and a multifunctional button illuminates. -
Page 22: Pairing
PRESSURE UNIT and MESI TUBELESS CUFF UNIT devices need to PAIRING be paired with the MESI mTABLET UNIT. Take the MESI mTABLET UNIT and open Doctor’s profile (for more information about user accounts see the USER PROFILE Chapter of the MESI mTABLET Instruction Manual). - Page 23 The final screen indicates successful connection between the MESI mTABLET UNIT and the MESI TOE BLOOD PRESSURE UNIT and MESI TUBELESS CUFF UNIT devices. It is possible to access additional information about the connected modules in User Profile >Settings >...
-
Page 24: Attaching The Toe Blood Pressure Cable To The Unit
NOTE During transportation, the toe blood pressure cable should be disconnected from the MESI TOE BLOOD PRESSURE UNIT. To detach the toe blood pres- sure cable, push the side buttons on the connector and pull it out from the MESI TOE BLOOD PRESSURE UNIT. -
Page 25: Attaching The Arm Cuffs To The Unit
MESI TUBELESS ATTACHING THE ARM CUFFS TO CUFF UNIT. The conical cuff can be oriented any way to the MESI THE UNIT TUBELESS CUFF UNIT. To connect the cuff, follow the next steps:... -
Page 26: Detaching The Arm Cuffs To The Unit
DETAILED INSTRUCTIONS 5.1.8 During transportation, each conical cuff should be disconnected DETACHING THE from the MESI TUBELESS CUFF UNIT. To detach the cuff, follow ARM CUFFS TO the steps listed below: THE UNIT Step 1 Step 2 Firmly hold the MESI TUBELESS CUFF UNIT Push the sliding lock in the indicated your hands. -
Page 27: Patient Selection
DETAILED INSTRUCTIONS 5.2 PATIENT SELECTION Before performing a measurement, a patient needs to be selected or added to your working groups patient list. 5.2.1 SELECTING A PATIENT Step 1 Step 2 Step 3 Press the patient tab button Use the search bar (1) or Select the patient. -
Page 28: Addinga Patient
DETAILED INSTRUCTIONS 5.2.2 ADDING A PATIENT Step 1 Step 2 Step 3 On your home screen Fill out the required fields Save the patient by pressing press the button in the (Name, Surname, Date of button. Patient tab. Birth and Sex) and any additional information regarding the patient. -
Page 29: Cuff Placement
2 arm cuffs. The TBI measurement can be made using 4 or 3 CUFF PLACEMENT cuffs. When using 4 cuffs the MESI mTABLET TBI System uses the SmartArm algorithm to identify the arm with the higher systolic blood pressure, which can be used in the TBI calculation. - Page 30 DETAILED INSTRUCTIONS Option 1: TBI Measurement with 3 Cuffs (Right Arm) Red cuff on right arm Black cuff on Green cuff on right toe left toe Option 2: TBI Measurement with 3 Cuffs (Left Arm) Yellow cuff on left arm Black cuff on Green cuff on right toe...
- Page 31 DETAILED INSTRUCTIONS Position the cuff 1-2 cm above the elbow joint. Align the artery label with the artery on inner side of an arm. PLACEMENT – PLACING THE CUFF ON THE Place the cuff so that there is two fingers’ width of room between the limb and the cuff.
- Page 32 Probe should not create any additional pressure on the pulp of the toe. NOTE Please read the Instructions for Use for other available MESI TUBELESS CUFF UNITS or contact your local distributor for more information. NOTE...
-
Page 33: Performing A Tbi Measurement
PERFORMING THE TBI MEASUREMENT Step 1 Step 2 Step 3 On your MESI mTABLET After selecting the patient, Select 3 cuff or 4 cuff meas- UNIT, select an existing please select the TBI meas- urement (when choosing patient or add a new one. - Page 34 DETAILED INSTRUCTIONS NOTE If the MESI TUBELESS CUFF UNIT or MESI TOE BLOOD PRESSURE UNIT have low batteries or connectivity issues, a notification will be displayed on the instructions screen. In case of errors please consult Chapters 8 ERRORS and 9 TROUBLESHOOTING.
- Page 35 DETAILED INSTRUCTIONS Step 7 Step 8 Check the signal and wait for the PPG wave- During the measurement the arm pressure forms of each toe to stabilise. You can amplify waveforms show pressure oscillation for each the signal strength by pressing the - (1) and + (2) arm while the pressure next to the oscillation buttons or wait a few moments for the device to displays the current pressure in the corre-...
-
Page 36: Reviewing A Tbi Measurement
DETAILED INSTRUCTIONS 5.4 REVIEWING A TBI MEASUREMENT Go to: previous/next result Repeat the measurement Share for second opinion Print result Navigation Area Go to: Application screen Discard result Right and Left Toe-Brachial Index Measurement Heart rate information SmartArm™ automatic higher blood pressure result Show more: right and left toe pressure, right and left... -
Page 37: Mesi Mtablet Results Screen
DETAILED INSTRUCTIONS 5.4.1 When the measurement process is completed the result will be MESI mTABLET displayed on the screen. It consists of five different areas: Navigation RESULTS SCREEN Area, Measurement Parameters (Right/Left Toe Brachial Index, Systolic/Diastolic Pressure, Heart Rate), PPG Pulse Waveform Recordings, Oscillation Graphs and Pulse wave Forms, patient’s... -
Page 38: Comments Area
If the results of the Toe-Brachial Pressure Index measurement are very unusual, repeat the measurement three times. 5.5.2 The MESI mTABLET TBI uses the unique FirstWave™ algorithm PPG PULSE to automatically detect the first returning pulse waveform which WAVEFORMS indicates systolic blood pressure in the toes. - Page 39 DETAILED INSTRUCTIONS Right after reaching the maximum pressure, all four (selected) cuffs will start deflating simultaneously at a controlled rate. At some point in the deflation phase the blood flow in toes will return and waveforms will re-establish. The very first returning waveform represents the point of systolic blood pressure in the toe.
-
Page 40: Multifunctional Button
DETAILED INSTRUCTIONS 5.6 MULTIFUNCTIONAL BUTTONS 5.6.1 Even though control of most of the MESI TOE BLOOD PRESSURE LED INDICATORS UNIT and MESI TUBELESS CUFF UNIT functions is performed through the MESI mTABLET UNIT interface, there is still a multifunctional button on top of the devices. Next to the basic colour light notification functionalities of this button there is also a possibility to perform some additional operations. -
Page 41: Standby
5.6.1.2 Green The battery is fully charged. Charging Orange The unit is charging. 5.6.1.3 The unit is waiting for confirmation from the MESI Blue Pairing mTABLET UNIT. 5.6.2 BUTTON FUNCTIONS 5.6.2.1 Status Check... -
Page 42: Maintenance
>200 measurements or 3.5 hours of continuous use. To charge the battery, place the modules on the MESI LARGE CHARGING PLATE UNIT. During the charging process the light on the module will be flashing yellow. Only when the device is completely charged it will start flashing green. - Page 43 • Use a soft lint-free cloth dampened with a proper cleaning agent to clean and disinfect the exterior/interior of the MESI TOE BLOOD PRESSURE UNIT/MESI TUBELESS CUFF UNIT.
-
Page 44: Disinfection
NOTE In the event of mechanical stress, the device must be calibrated! 6.3 DISINFECTION MESI TOE BLOOD PRESSURE UNIT/MESI TUBELESS CUFF UNIT: For disinfection use commercially available disinfectants intended for a professional healthcare environment. Refer to the producer's Instructions for Use. -
Page 45: Product Life And Storage
The MESI mTABLET TBI device users must be adequately trained in use of the device. This training must be performed by a trained MESI GENERAL representative. Prior to initial use of the device users must read the... -
Page 46: Measurement Procedure
Do not use the device when it is wet. After cleaning the device with a damp cloth, wait for it to dry. Only use the device when it is completely dry. The MESI mTABLET TBI is not intended for use in conjunction with high- frequency surgical equipment. -
Page 47: Maintenance
GENERAL WARNINGS Important information on electromagnetic compatibility (EMC). As the number of electronic devices such as computers and mobile phones in the room increases, medical devices can become sensitive to the electromagnetic influences of other devices. Electromagnetic interference can cause medical devices to malfunction, which can potentially lead to dangerous situations. -
Page 48: Errors
The device is class A equipment and can cause radio interference or even cause nearby devices to cease to function. It may be necessary to reposition the MESI mTABLET TBI device or protect the room containing the device from electromagnetic radiation. - Page 49 TBI UNIT/MESI TOE BLOOD application. Connection between Remove any mobile devices PRESSURE UNIT not in the main unit and MESI in proximity of the main unit range. mTABLET UNIT is not or bring the MESI mTABLET optimal.
-
Page 50: Troubleshooting
Use cuffs of the correct size. Continuously Incorrect state of the Press and hold the button on flashing either diagnostic module. top of the MESI TOE BLOOD purple or red. PRESSURE UNIT for 15 seconds to restart it. TBPMD unable to Incorrect state of the... -
Page 51: Standard Compliance
STANDARD COMPLIANCE The provisions of Council Directive 93/42/EEC concerning medical devices were complied with. The standards in the table below were complied with. STANDARD Reference Number Description (ID:year) COMPLIANCE EN 60601-1:2006 Medical electrical equipment - Part 1: /A1:2013 General requirements for basic safety and essential performance EN 60601-1-2:2015 Medical electrical equipment - Part 1-2:... -
Page 52: Manufacturer Declaration On Emc
11.1 MANUFACTURER DECLARATION ON EMC MESI mTABLET TBI is intended for use in the electromagnetic environment specified below. The customer or the user of the above listed models should ensure that they are used in such an environment. Emissions Test... - Page 53 STANDARD COMPLIANCE MESI mTABLET TBI is intended for use in the electromagnetic environment specified below. The customer or user of the models listed above should ensure that they are used in such an environment. Patient Coupling PORT IEC 60601 Electromagnetic Environment -...
- Page 54 STANDARD COMPLIANCE MESI mTABLET TBI is intended for use in the electromagnetic environment specified below. The customer or user of the models listed above should ensure that they are used in such an environment. Enclosure Port IEC 60601 Electromagnetic Immunity Test...
- Page 55 STANDARD COMPLIANCE MESI mTABLET TBI is intended for use in the electromagnetic environment specified below. The customer or user of the models listed above should ensure that they are used in such an environment. Input AC Power PORT IEC 60601...
- Page 56 STANDARD COMPLIANCE The MESI mTABLET TBI is intended for use in the electromagnetic environment specified below. The customer or user of the models listed above should ensure that they are used in such an environment. Signal Input/Output Parts PORT IEC 60601...
-
Page 57: Essential Performance
STANDARD COMPLIANCE/IMPORTANT LABELS 11.2 ESSENTIAL PERFORMANCE MESI TBI MODULE is a part of MESI mTABLET TBI whose essential performance is performing a TBI measurement in specified oper- ating conditions. The measurement is defined as a measurement process and data storage to a MESI mTABLET UNIT. Due to the device's high sensitivity, intended use and operating modes, the device is susceptible to EM interferences. -
Page 58: Notes
NOTES... - Page 59 NOTES...
- Page 60 11-2020/V. 1.2...






Need help?
Do you have a question about the mTABLET TBI and is the answer not in the manual?
Questions and answers